HERE - UNH Center for Freshwater Biology
advertisement

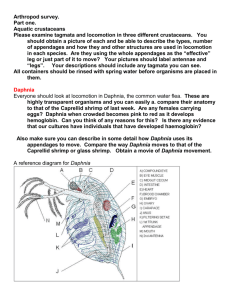
Distinguishing Characteristics Proximal, middle, and distal pecten of postabdominal claw all the same size. Ocellus present. Swimming hair at the base of the second segment of the three-segmented ramus extends beyond tip of ramus. Swimming hairs of antennae do not extend beyond posterior margins of valves. Small head and valves, no more than 1.3 mm in length. Head often with small anterior point during summer. Similar Species Commonly confused with D. parvula. Geographic Distribution Daphnia ambigua occurs in Europe and North and South America. Populations of D. ambigua have been seen in Mexico, the Central United States,the Appalachian range, the Atlantic and Pacific coasts. (9) Reported Habitats Curiously, D. ambigua is often found in cool deep water in the lower thermocline, suggesting a tolerance to cold temperatures, but on the other hand it is one of the most common Daphnia species found in tropical and sub tropical regions. Food & feeding behavior Daphnia ambigua are the smallest daphnia species in North America. Even with increased food concentrations, the maximum attainable length of Daphnia ambigua is less than half that of D. pulex. D. ambigua’s small size has been hypothesized to be due to either size selective predation or resources allocated toward predation defense such as a thicker carapace. (5) Reproductive Habits Daphnia ambigua showed optimal reproductive capability from 25-30°C. (6) Predation In the laboratory setting, one-day old Daphnia ambigua were eaten by Mesocyclops leuckarti. Larger daphnia not eaten were injured by copepods trying to eat them. (1) Daphnia ambigua is the smallest Daphnia species, rarely exceeding 1 mm in body length. Its small size makes it especially vulnerable to predation by Chaoborus larvae. In response to Chaoborus kairomones, or chemical given off by Chaoborus, adults & juvenile D. ambigua often form a sharp pointed helmet on the central axis of the head, typically seen in mid to late summer. exposed to Chaoborus larvae or larvae extract exhibited helmets. Adults & older young had significanlty shorter helmets or no helmet at all. (2) Helmets are only induced when juveniles are directly exposed to the Chaoborus factor; if only exposed to Chaoborus factor as eggs, helmets will not develop in the juvenile stages. (3) At lower food levels, D. ambigua that have been exposed to Chaoborus and grown helmets have reduced growth, survival and reproductive rates than D. ambigua not exposed to Chaoborus. (4) Competition In a laboratory setting, Daphnia ambigua excluded the rotifer Keratella cochlearis in high food concentrations and was able to survive starvation much longer than the rotifer (8). D. ambigua has been shown to suppress ciliate populations by predation and interference competition and could possibly be a trophic link between nanoplankton and metazon zooplankton (10). Migration Daphnia ambigua in monomictic, eutrophic lake El Plateado, Chile, migrated vertically with the greatest amplitude of migration observed in the warm season(7). Seasonal Variations The D. ambigua population in lake El Plateado, Chile, showed seasonal variation, being common in the winter and absent from the water column in the summer. (7) D. ambigua populations in North American lakes are largest in the spring (11). (2) Adults & young exposed to Chaoborus larvae or larvae extract exhibited helmets. (NOTE: Put this picture in at the words “sharp pointed helmet” in the Predation section) (5) (NOTE: Put this picture in at the words “maximum attainable length ” in the Food & Feeding Behavior section) (1) CHANG, K. H. and T. HANAZATO. (2003) Vulnerability of cladoceran species to predation by the copepod Mesocyclops leuckarti: laboratory observations on the behavioural interactions between predator and prey. Freshwater Biology. 48: 476-484. (2) HEBERT, P.D. and P.M. GREWE. (1985). Chaoborus-induced shifts in the morphology of Daphnia ambigua. Limnol. Oceanogr. 30: 1291–1297. (3) HANAZATO, T. (1990) Induction of helmet development by a Chaoborus factor in Daphnia ambigua during juvenile stages. Journal of Plankton Research. 12:12871294. (4) HANAZATO, T. (1991) Influence of food density on the effects of a Chaoborus released chemical on Daphnia ambigua . Freshwater biology. 25: 477-483. (5) LYNCH, M. (1992) The Life History Consequences of Resource Depression in Ceriodaphnia Quadrangula and Daphnia Ambigua. Ecology. 73:1620-1629. (6) MALLIN, M.A. and W.E. PARTIN, WE (1989) Thermal Tolerances of Common Cladocera. Journal of Freshwater Ecology 5: 45-50. (7) RAMOS-JILIBERTO, R. and L. R. ZÚÑIGA. (2001) Depth-selection patterns and diel vertical migration of Daphnia ambigua (Crustacea: Cladocera) in lake El Plateado. Revista Chilena de Historia Natural 74:573-585. (8) MACISAAC, H.J., and J.J. GILBERT. (1991) Competition between Keratella cochlearis and Daphnia ambigua : effects of temporal pattern of food supply. Freshwater biology. 25: 189-198. (9) HEBERT, P.D., J.D. WITT, and S.J. ADAMOWICZ. (2003) Phylogeographical patterning in Daphnia ambigua: Regional divergence and intercontinental cohesion. Limnology and Oceanography. 48: 261-268. (10) WICKHAM, S.A., and J.J. GILBERT. (1993) The comparative importance of competition and predation by Daphnia on ciliated protists. Archiv fur Hydrobiologie. 126: 289-313. (11) ALLAN, JD. (1977) An Analysis of Seasonal Dynamics of a Mixed Population of Daphnia, and the Associated Cladoceran Community. Freshwater Biology 7: 505512.