SOP FOR HP1 - Association for Organics Recycling
advertisement

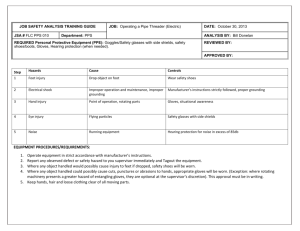
Folder: STANDARD OPERATING PROCEDURE Z/004 The determination of ammonium in organic wastes (liquid or solid) Edition: 05 Last amended 31.01.2006 Authorised by: I Morris Page 1 of 5 Date: INTRODUCTION NH4-N is extracted from organic waste samples with 2M KCl. Samples appearing to have less than 10% solids are routinely treated as liquids, samples appearing to have above 10% solids are routinely treated as solids. An aliquot of the extract is made alkaline and the released ammonia is determined titrimetrically, after removal by distillation. REFERENCE DOCUMENTS RB427 The Analysis of Agricultural Materials1985. Method 53. SOP H/007 – Determination of residual moisture in plant material and animal feedingstuffs SOP Z/002 – Determination of nitrogen/crude protein content using the Kjeldahl technique SOP SAMP/006 – Procedures for handling potentially biohazardous materials MATERIALS AND EQUIPMENT 1. Reagents 1.1 2M KCl. Dissolve 149.2 0.1g of KCl in 1 L of water. Store for up to three months at room temperature. 1.2 NH4-N Standard Solution, 1.4 mg/ml. Dry ammonium sulphate at 102 2°C for one hour 5 min and cool to room temperature in a desiccator. Dissolve 3.3035 0.001g of the dried salt in deionised water and dilute to 500ml with deionised water. Store for up to three months at room temperature. 1.3 Boric Acid Solution, approx. 1% m/v. Store for up to four months at room temperature. 1.4 Magnesium Oxide Powder (MgO) 1.5 Methyl Red Solution. Dissolve 1.0 0.01g of methyl red in 1L of methanol. Store at room temperature for up to two months. 1.6 Bromocresol Green Solution. Dissolve 1.0 0.01g of bromocresol green in 1L of methanol. Store at room temperature for up to two months. 1.7 Sulphuric Acid, 50 mM volumetric solution, available from VWR. Store for up to six months at room temperature. Z/004 2. Edition: 05 Page 2 of 5 Equipment 2.1 Bottles - 250 ml, wide mouth with screw caps. 2.2 Kjeldahl Tubes, 250ml and 500ml 2.3 Shaking machine - The Griffin bottle shaker (A. Gallenkamp and Co. Ltd., Cat No. SGL - 200) is suitable. 2.4 Distillation units, Kjeltec 2300 Auto analyser for direct steam distillation. PROCEDURES 1. Sampling Non-food, non-soil samples are prepared according to SOP SAMP/015 2. Sample Extraction Samples are extracted with 2M KCL prior to distillation. Some samples can be distilled directly. 2.1 The extraction is usually carried out in the sample preparation section and the extract transferred to the Kjeldahl unit as per SOP SAMP/005. 2.1.1 Transfer 20 0.1g of fresh solid waste or 20 0.1ml of an organic liquid waste into a bottle. Add 100 2ml of 2M KCl. If the sample size is small then it is acceptable to weigh out 10g and use 50ml of extractant. 2.1.2 Cap the bottle and place on the shaking machine. Shake for two hours 10 min. Filter through a 15 cm Whatman No. 541 filter paper and retain the filtrate for the determination of ammonium - nitrogen. 2.1.3 Transfer to the Kjeldahl section. 2.2 3. Carry out a blank determination. Distillation 20ml of the extract from step 2.1.2 is taken for distillation. 3.1 Transfer the required volume of extract to a 250ml Kjeldahl tube. in Table 1 below. Volumes are taken to ±0.01ml and the volume is recorded. 3.2 Add approximately 0.5g of MgO and wash this down the neck of the tube with deionised water. The total volume of the tube should be between 20ml and 150ml. Z/004 4. Edition: 05 Page 3 of 3.3 Certain samples will froth excessively on addition of MgO. If this occurs repeat the process using a 500ml Kjeldahl tube. 3.4 Transfer the tube to the Kjeltec 2300 analyser and distil the samples following the instructions in the Kjeltec manual, using the pre-set programme, Kjel3 3.5 Run blanks between samples to avoid carry over from high samples 5 Calculation of result Standard Solution (1.2) = 1.4 mg/ml NH4N 5ml of Standard Solution gives a titre of 5ml So, 1ml of titre ≡ 1.4mg NH4N concentrat ion of NH4 N in the tube titre 1.4 Det Calculation NH4N for extracted solids Titre x 1.4 x ([100 + wt] – [DM%/100 x wt]) x 1000 V1 x wt Reporting Units mg/Kg SAR NH4N for Titre x 1.4 x ([100 + wt] – [DM%/100 x wt]) x 1000 x 100 mg/Kg DM extracted solids to V1 x wt %DM be reported on a dry matter basis NH4N for mg/L SAR Titre x 1.4 x ([100 + V2] – [DM%/100 x V2]) x 1000 extracted liquids V1 x V2 Where V1 = the volume taken for distillation V2 = the volume of liquid sample taken for extraction Z/004 5. Edition: 05 Page 4 of 5 AQC An AQC duplicate is analysed on a basis of 1 per 10 samples. If the batch is smaller than the AQC frequency should be one per batch. 6. Safety The Kjeldahl methods and procedures are potentially very hazardous and are carried out in a corrosive environment. Neoprene gloves and gauntlets are provided together with facial visors. Gloves should be worn at all times because of possible dermatological effects of tablets and even dilute acids and alkalis. Disposable gloves are used for sample handling and weighing out. Additional safety extraction fans have been fitted in the digestion room in case of failure of the main extraction fan. The main fan needs to be switched on before samples have acid added. To avoid noxious fumes from cold acid, ensure the room is well ventilated. Clothing, especially jeans can become spotted with holes from splashes of dilute acid. In case of accidental acid drenching, an emergency shower is provided in the digestion room. This shower should be tested every week to ensure it continues to be in good working order. 6.1 Chemical hazard data sheet Chemical Kjeltabs Hazard May be harmful if ingested in quantity Dust may irritate eyes Control Measure Good ventilation Gloves Safety glasses Occupational Exposure Standard – not assigned Causes severe burns to eyes and skin Fume cupboard Sulphuric If ingested causes severe internal irritation and Gloves - nitrile Acid damage Safety glasses Dilute acid irritates the eyes and skin and may cause Face shield burns and dermatitis Occupational Exposure Standard – 1 mg/m3 per 8 hour Corrosive to body tissue causing burns and severe Gloves Sodium ulceration Safety glasses hydroxide Irritant and harmful as mist or spray solution If ingested causes severe internal irritation or damage Occupational Exposure Standard - 2 mg/m3 NaOH per 8 hour May be harmful if ingested in quantity, causing Respirator Ammonium nausea, vomiting and diarrhoea Gloves Sulphate May irritate eyes and respiratory system if inhaled as Safety glasses a dust Occupational Exposure Standard - not assigned May be harmful if ingested in quantity Gloves Methyl Red Irritating to eyes Safety glasses Stains Occupational Exposure Standard - not assigned Gloves Bromocresol May be harmful if ingested in quantity Irritating to eyes Safety glasses Green Stains Occupational Exposure Standard - not assigned Z/004 Edition: 05 6.1 Page 5 of 5 Chemical hazard data sheet (continued) Chemical Methanol Hazard Control Measure Solvent room or fume cupboard Gloves Safety glasses Avoid sources of ignition. Highly flammable Vapour/air mixture explosive Toxic by ingestion Damaging if splashed in eyes High concentrations of vapour may cause dizziness, stupor, cramps and digestive disturbance. Lower levels may cause headache and nausea. Chronic effects - damages the central nervous system, particularly the optic nerve and internal organs. Can react vigorously with oxidising materials Can react vigorously with chloroform in the presence of sodium and sodium hydroxide Avoid contact with oxidisers. Occupational Exposure Standard - 260 mg/m3 per 8 hour Boric Acid May be harmful if ingested in quantity Gloves May be irritating to eyes Safety glasses Occupational Exposure Standard - not assigned Chromium Harmful by ingestion and if inhaled as a dust Dust mask Metal Powder May be irritating to eyes, skin and respiratory Safety glasses system Gloves May cause nasal and skin ulceration Prolonged exposure to dust or fumes may cause vomiting, diarrhoea, liver and kidney damage and stomach pains Occupational Exposure Standard – 0.5 mg/m3 per 8 hour Sucrose Ingestion of significant quantities may lead to Safety glasses metabolic imbalances Gloves May irritate eyes Occupational Exposure Standard - 10 mg/m3 per 8 hour Hydrochloric Causes severe burns to eyes and skin Fume cupboard Acid If ingested causes severe internal irritation and Gloves damage Safety glasses Extremely irritating, harmful vapour Occupational Exposure Standard - 7 mg/m3 per 8 hour