jcb_23244_sm_SupplMaterial
advertisement

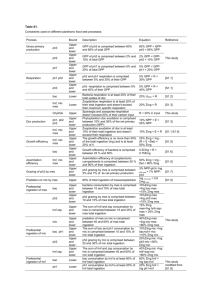
S1 Text GENERATION OF OED5 AND OEE CELLS Mouse ES D3 cells infected by the viral produced from plasmid, pCS-UBC-Dusp5, to produce cells that over-expressing the Dusp5 protein (OED5 cells), and mES D3 cells over-expressing the EGFP protein (OEE cells) were obtained by the same strategy, for use as a control. In order to determine whether the exogenous Dusp5 mRNA was transcribed, the following pair of PCR primers was designed: a primer with mDUSP5F5 located at the 3’ terminus of Dusp5 and a primer with PCSR located in the 3’ untranslated region (UTR) derived from the plasmid (Table 1). Following transfection, OED5, OEE and wild type D3 cells (WT cells) were passaged for two generations before harvesting to eliminate the plasmid and viral contaminants. The PCR amplified band only appeared in the OED5 cell sample (Fig. S1A), suggesting that exogenous Dusp5 mRNA was transcribed only in OED5 cells. To assess this result quantitatively, qRT-PCR analysis was performed and the results demonstrated that the Dusp5 expression level in OED5 cells was up-regulated almost five-fold compared with OEE and WT cells (Fig. S1B). This result was confirmed by Western blot analysis with a Dusp5 specific antibody (Figs S1C and D). GENERATION OF DUSP5 KNOCKDOWN CELLS For the knockdown of Dusp5, seven small hairpin RNAs (shRNAs) with stem sequences complementary to mouse Dusp5 mRNA were selected. However, of the seven, only shDusp5Mu1 (Target Sequence: GCTGACATTAGCTCCCACTTT) showed efficient knockdown of the target gene. Mouse ES cells infected with shDusp5Mu1 (SHD5-pool cells) showed a twofold reduction in Dusp5 expression compared to WT cells and control cells (scramble shRNA-treated D3 cells (Scr cells)) (Fig. S2A). Western blot analysis with a Dusp5 specific antibody revealed weaker immunoblot signals in SHD5-pool cells demonstrating a decrease in the amount of Dusp5 protein (Fig. S2B). Analysis of the two isolated clones obtained from Dusp5 knockdown cells (SHD5-e5, SHD5-g2) yielded similar results (Fig. S2A-B). Fig S1. Over-expression of Dusp5 in mES cells. A: RT-PCR analysis of exogenous Dusp5 expression in WT, OEE and OED5 cells. Gapdh was amplified as the internal control. Mock: no template control. B: Over-expression of Dusp5 in WT, OEE, OED5 cells were analyzed by qRT-PCR (n=4). Gene expression was normalized against a ß-actin internal control. Error bar represents the means ±S.D. from four independent experiments. **P<0.01. C: Western blot analysis of WT, OEE and OED5 cells with an anti-Dusp5 antibody and an anti-ß-actin antibody. Immunoblot signals are indicated by arrows. D: Quantitation of the Dusp5 band intensity in the immunoblot in C was conducted using ImageJ 1.44 program (National Institute of Mental Health, Bethesda, Maryland, USA). The densitometric intensity of the Dusp5 band was normalized against that of ß-actin, and corresponding values of OEE and OED5 were expressed as fold numbers of that of WT. WT: wild type mES D3 cells; OEE: mES D3 cells over-expressing the EGFP protein; OED5: mES D3 cells over-expressing the Dusp5 protein. Fig S2. Dusp5 knockdown in mES cells. A: Knockdown performance of shDUSP5Mu1 in mES cells by qRT-PCR (n=4). Gene expression was normalized against a ß-actin internal control. Error bar represents the means ±S.D. from four independent experiments. *P<0.05. B: Western blot analysis of mES cells with an anti-DUSP5 antibody and an anti-Gapdh antibody. Immunoblot signals are indicated by arrows. WT: wild type mES D3 cells; Scr: scramble shRNA-treated mES D3 cells; pool: SHD5-pool cells; g2: SHD5-g2 cells; e5: SHD5-e5 cells. Fig S3. Dusp5 expression was induced by bFGF treatment. Mouse ES cells were plated on gelatin-coated tissue culture plates over night, before addition of bFGF (10 ng/ml). Cells were then harvested for RNA isolation, and followed by qRT-PCR analysis (n=4). Gene expression was normalized against a ß-actin internal control. Error bar represents the means ±S.D. from four independent experiments. *P<0.01. Fig S4. The role of Dusp5 in mES pluripotency and differentiation Undifferentiated mES cells employed transcription factors, such as Nanog and Oct4, and various cytokines such as LIF, to maintain the pluripotent state. The commitment of mES cells to differentiation is induced by cell signaling molecules, such as phospho-Erk. Dusp5 counteracts differentiation by inhibiting FGF/Erk signaling. During EB development, Dusp5 may be important for endoderm and mesoderm differentiation, via the precise control of Erk signaling.