TABLE 5. Yeast: Percent Susceptible
advertisement

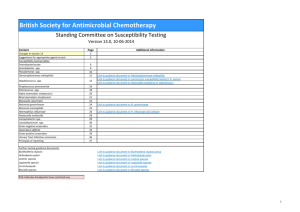
Clinical Microbiology Laboratory Durham, North Carolina 27710 SUMMARY OF ANTIMICROBIAL SUSCEPTIBILITY TEST RESULTS 1999 HOURS OF OPERATION: 24 hours per day 365 days per year. TELEPHONE: 684-2089 A certified medical technologist is on duty at all times. CONSULTATIONS: Page 970-8885 A physician (Medical Microbiology Fellow or Resident) is on call at all times with faculty backup. TEACHING: Teaching Rounds (Clinical correlations) Monday-Friday 1:15-1:45 PM 2/2000 These data were obtained by broth microdilution or disk diffusion methods according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines. Data are based on microorganisms from both inpatients and outpatients. No attempt was made to differentiate nosocomial isolates. TABLE 1. Percent Susceptiblea, Gram-positive Cocci (MIC breakpoint, g per ml) Beta-lactams Other Antimicrobials ________________________ ________________________________ Microorganism (No. tested) AMP NAF CFZ CLI ERY VAN T/S (8) (2) (8) (0.5) (0.5) (4) (2/38) ________________________________________________________________________________________________________________________ Enterococcusb (1084) 82 NT NT NT NT 79 NT Staphylococcus aureus (2017) NT 49 49 54 37 100 77 Staphylococcus, coagulasenegative (341) NT 26c 26 NT NT 100 47 ________________________________________________________________________________________________________________________ a Susceptible implies that an infection due to the microorganism may be appropriately treated with the dosage of antimicrobial agent recommended for that type of infection and infecting species, unless otherwise contraindicated. b For Enterococcus the designation "susceptible" implies the need for combined therapy (ampicillin or vancomycin plus an aminoglycoside) in endocarditis or other serious invasive infections to achieve bactericidal action and an improved therapeutic response. c Susceptible MIC breakpoint is <0.25 µg/ml for coagulase-negative Staphylococcus. MIC (minimum inhibitory concentration) is the lowest concentration of a drug which will inhibit growth of a microorganism in vitro. For the drug to be effective in vivo, a higher concentration than the MIC of the drug (at least 2 to 4 times higher) should be achieved at the site of infection. MIC breakpoints are based on achievable serum levels in adults with normal renal function. ________________________________________________________________________________________________________________________ NT = Not tested. $ = Relative daily acquisition cost for recommended doses of parenteral therapy. AMK (amikacin) - $/$$) AMP (ampicillin - $) AMP/SUL (ampicillin/sulbactam - $$$$$) CAZ (ceftazidime - $$$) CFZ (cefazolin - $) CIP (ciprofloxacin) (oral - $; IV - $$$$) CLI (clindamycin - $) CRO (ceftriaxone - $$$) CTX (cefotaxime - $$$) ERY (erythromycin - $) GEN (gentamicin - $) IMP (imipenem - $$$$$$) NAF (nafcillin - $) PEN (penicillin - $) PIP (piperacillin - $$$$$) TOB (tobramycin - $) T/S (trimethoprim/sulfamethoxazole - $) VAN (vancomycin - $$) ________________________________________________________________________________________________________________________ TABLE 2. Percent Susceptible, Gram-negative Bacilli (MIC breakpoint, g per ml) Other Beta-lactams Aminoglycosides Antimicrobials __________________________________________ ___________________ ______________ AMP/ Microorganism (No. tested) AMP SUL CFZ CAZ CROb IMP PIP GEN AMK TOB CIP T/S (8) (8/4) (8) (8) (8) (4) (16) (4) (16) (4) (1) (2/38) _______________________________________________________________________________________________________________________ Acinetobacter baumannii (71) NT NT 0 90 74 100 83 85 93 89 88 84 0 98 98 100 100 100 93 100 100 100 100 98 13 58 7 61 62 100 55 95 100 95 91 80 Enterobacter aerogenes (146) 0 0 0 76 77 99 74 97 98 97 90 94 Enterobacter cloacae (246) 0 0 0 67 65 100 64 95 100 96 93 89 Escherichia coli (2593) 59 73 91 99 99 100 63 97 100 98 97 82 Klebsiella oxytoca (76) 0 76 55 89 91 100 85 89 94 91 95 89 Klebsiella pneumoniae (764) 0 75 86 89 90 100 72 89 97 89 87 77 Morganella morganii (46) 0 0 0 84 95 97 82 92 97 92 93 91 Proteus mirabilis (306) 91 96 94 99 100 98 95 95 99 97 88 91 Pseudomonas aeruginosa (1105) NT NT NT 80a NT 79 82c 67 75 85 66 11 0 0 0 96 99 100 95 99 99 92 91 92 NT NT 0 39 0 0 0 0 0 0 31 94 Citrobacter koserii (diversus) (56) Citrobacter freundii (98) Serratia marcescens (142) Stenotrophomonas maltophilia (123) _______________________________________________________________________________________________________________________ Numbers in boldface indicate >10% decrease in susceptibility from 1998 to 1999 a For P. aeruginosa, ceftazidime (CAZ) usually predicts susceptibility to aztreonam. b For Enterobacteriaceae, ceftriaxone (CRO) usually predicts susceptibility to cefotaxime. c For P. aeruginosa, susceptible MIC breakpoint for piperacillin (with or without tazobactam) is < 64 µg/ml which requires high doses. TABLE 3. Percent Susceptible, Anaerobic Gram-negative Bacillia MIC Antimicrobial Breakpoint B. fragilis groupb (cost per day) (µg per ml) (No. tested = 106) _________________________________________________________________ Ampicillin/sulbactam ($$$$$) 8/4 86 Cefoxitin ($$$) 16 67 Clindamycin ($) 2 75 Imipenem (Meropenem) ($$$$$$) 4 100 Metronidazole ($ - $$) 4 100 Piperacillin/tazobactam ($$$$$) 32/4 100 __________________________________________________________________ a B. melaninogenica group, Fusobacterium spp., and gram-positive anaerobic cocci and bacilli are usually susceptible to -lactam antibiotics, clindamycin, and metronidazole. b Includes B. fragilis, B. thetaiotaomicron, B. ovatus, B. distasonis, B. vulgatus, B. uniformis, and B. caccae. TABLE 4. Fastidious Microorganisms 1. Haemophilus influenzae (No. tested = 142). All isolates except one were non-type b. 89 (63%) were ß-lactamase negative and predictably susceptible to ampicillin (amoxicillin); 53 (37%) were ß -lactamase positive and, therefore, resistant to ampicillin (amoxicillin). H. influenzae is predictably susceptible to cefotaxime and ceftriaxone and usually to cefuroxime. 2. Streptococcus pneumoniae. Rx NOTE: Breakpoints for pneumococci are based on concentrations of penicillin and ceftriaxone (or cefotaxime) required to treat meningitis. Infections in other sites usually respond to these drugs of choice for pneumococcal infections despite in-vitro resistance based on meningitis breakpoints. Consult Infectious Diseases if questions about individual patients. (No. tested) Penicillin (121) Ceftriaxone or cefotaxime (121) Erythromycin (93) MIC Breakpoint (µg/ml) % Susceptible % Intermediate 16 (0.1-1) 45 (<0.06) 24 (1) 64 (<0.5) 46 (<0.25) ---- % Resistant 39 (>2) 12 (>2) ---- TABLE 5. Yeast: Percent Susceptible (No. tested) Amphotericin B* (MIC Breakpoint = <1 g/ml) Fluconazole (MIC Breakpoint = <8 g/ml) Candida albicans 100 (35) 78 (51) C. tropicalis 100 (14) 78 (41) C. parapsilosis 100 (19) 91 (34) C. krusei l m C. glabrata 100 (14) 15 (20) C. lusitaniae l Cryptococcus neoformans l 88 (16) l = usually susceptible = often resistant m = inherently resistant Testing is useful in serious or persistent infections due to organisms with unpredictable susceptibility profiles, especially when standard regimens fail or are contraindicated. * NCCLS M27-A (1997) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (tentative breakpoint)