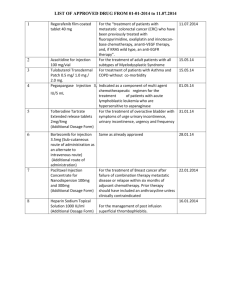
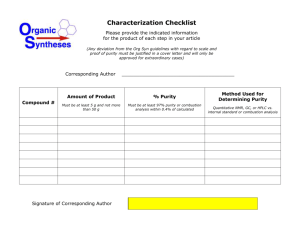
Batch # FAY-______ Date
advertisement

[18F]FALLYPRIDE FOR INJECTION: QUALITY CONTROL RECORD PET Radiopharmaceutical Sciences Section, Molecular Imaging Branch, National Institute of Mental Health, National Institutes of Health, Bldg. 10, Rm. B3 C338, Bethesda, MD 20892 Date of review: 05/17/04 Approved by:_____________________________ Initials___________ Date:_______ Victor W Pike, Ph.D Chief, PET Radiopharmaceutical Sciences, NIMH Batch # FAY-___________ Date________ Part I: Summary of QC Data Test Result 1. Vial integrity (Visual inspection) _______ 2. Solution free of particulates (Visual inspection) _______ 3. pH of the [18F]Fallypride for Injection (pH paper): Specification: (within range of 4.5−7.5) 4. pH: _______ Validation of analytical system (SOP # QA305) Column: Prodigy ODS (10 µm; 4.6 X 250 mm; 100 Å) Mobile phase: Acetonitrile-5 mM ammonium formate (50: 50 v/v) at 1 mL/min Absorbance detector: set at 305 nm Inject 20−50 ng of reference fallypride into HPLC system. The corresponding UV area is expected to be 100 ± 10% of UV area based on the calibration curve Rt =__________min. 5. Recovery: ________% Radiochemical purity (SOP # QA305) Specifications: ≥ 90% radiochemical purity _________% Volume of sample injected: 50−100 µL 6. Specific radioactivity (SR): Specification > 500 mCi/µmol at EOS ________mCi/µmol at ___________ 7. Chemical purity (SOP # QA305) Specifications: ≥ 90% chemical purity Rt =__________min. ___________% 8. Organic residues in [18F]Fallypride for Injection (SOP # QA304) Criteria: ≤ 0.04% (wt/Ʋ), acetonitrile; ≤ 10% (wt/Ʋ) dehydrated alcohol. Test completed on every 10th batch after release of drug product of [18F]Fallypride for Injection. Yes _______No ______ (see GC chromatogram and report) 9. Expiry time [18F]Fallypride for Injection expires at 4 h from EOS. 10. Membrane filter integrity test (SOP # GP104) Document 3. [18F]Fallypride for Injection: Quality Control Record Expiry time: ________________ Pass: Yes_______No ______ Page 1 of 4 [18F]FALLYPRIDE FOR INJECTION: QUALITY CONTROL RECORD PET Radiopharmaceutical Sciences Section, Molecular Imaging Branch, National Institute of Mental Health, National Institutes of Health, Bldg. 10, Rm. B3 C338, Bethesda, MD 20892 Date of review: 05/17/04 This batch has met all the quality control criteria: Chemist __________________________Initials__________________ Batch # FAY-____________ Date_____________ Date_____________ Part II: Quality Control and Radiochemistry Record (SOP # QA305) 1. Validation of HPLC system and Ionization Chamber Conditions for QC: Instruments used in QC: Column: Prodigy C-18 ODS (10 µm; 100 Å, 4.6 X 250 mm) Pump: Beckman 166 Mobile phase: Acetonitrile: ammonium formate (50: 50 v/v) Radioactivity detector: Bioscan Flow rate: 1 mL/min. UV Detector: Beckman UV detector wavelength: 305 nm (A) Validation of HPLC system: Inject 20−50 ng of reference fallypride into HPLC system. Amount injected ___________nmol; Retention time: ________ min Area of UV peak ____________ = _________nmol (standard curve). Recovery:________% (> 90%) (B) Validation of ionization chamber in hot-cell 5; see page 4. 2. Chemical Purity (≥ 90% based on UV area). Inject 50−100 L of [18F]Fallypride for Injection into HPLC system. See UV chromatogram. UV Area of fallypride __________________. Rt (fallypride) ___________min. Chemical purity of fallypride (excluding excipient peak(s))____________% Mass (nmol) = (UV Area (fallypride peak) - 614.01) / (355.024*364.45) = ____________nmol (based on the standard curve) 3. Radiochemical purity and specific radioactivity Inject 50−100 L of [18F]Fallypride for Injection into HPLC system (see 2). (A) Volume of [18F]Fallypride for Injection sample injected: ________µL (1) in saline ____ (2) in ethanol______ Pre-injection ___________µCi at ___________ Left in syringe __________µCi at _________ __= ___________µCi at ____ ____(decay corrected) Document 3. [18F]Fallypride for Injection: Quality Control Record Page 2 of 4 [18F]FALLYPRIDE FOR INJECTION: QUALITY CONTROL RECORD PET Radiopharmaceutical Sciences Section, Molecular Imaging Branch, National Institute of Mental Health, National Institutes of Health, Bldg. 10, Rm. B3 C338, Bethesda, MD 20892 Date of review: 05/17/04 Radioactivity injected ______________ µCi at _____________ (B) Radiochemical purity of [18F]Fallypride for Injection Rt ([18F]Fallypride) =____________min. Radiochemical purity ________________ % (Radioactivity channel in HPLC Chromatography) (C) Specific radioactivity (SR) calculation SR = __________________mCi/µmol at____________ = __________________mCi/µmol at____________ (EOS) =__________________ mCi/µmol at____________ (EOB) 4. Expiry time calculation This is calculated as the sooner of i) latest time that less than 2.0 µg (5.473 nmol) of Fallypride for Injection can be injected with a 5 mCi radioactivity dose, or ii) 4 h after EOS. The expiry time can be calculated from the following data: Volume of whole batch _______________mL. Radioactivity of whole batch: _________________mCi at____________ Radioconcentration _______________mCi/mL at __________________ The mass of fallypride in whole batch _______________µg This batch of [18F]Fallypride for Injection expires by _______________Batch # FAY-________ ___ _ Date:____________ 5. Analysis of organic residues (SOP # QA304 and see gas chromatogram) ≤ 0.04% (wt/v), acetonitrile, ≤ 10% (wt/v) dehydrated and alcohol. (Test completed on every 10th batch after release of drug product of [18F]Fallypride for Injection) Chemist __________________________Initials__________________ Batch # FAY-____________ Document 3. [18F]Fallypride for Injection: Quality Control Record ___________ Date_____________ Date_____________ Page 3 of 4 [18F]FALLYPRIDE FOR INJECTION: QUALITY CONTROL RECORD PET Radiopharmaceutical Sciences Section, Molecular Imaging Branch, National Institute of Mental Health, National Institutes of Health, Bldg. 10, Rm. B3 C338, Bethesda, MD 20892 Date of review: 05/17/04 Validation of ionization chamber in Hot-Cell 5 Cs-137 source reference # 970-31-4 204 μCi on 03/1/2003 Expected radioactivity on date of this test _____________ μCi on _____________ (use Excel Speadsheet on bench top HPLC computer) Acceptable range of radioactivity _____________to______________μCi. Measured radioactivity ____________ μCi on _____________(Remember to push Cs-137 button on dose calibrator, Bioscan Medical System) Does measured radioactivity fall within acceptable range (within 5% of expected value)? Yes or No (Circle one) Co-57 source reference # 970-32-8 204 μCi on 03/1/2003 Expected radioactivity on date of this test _____________ mCi on _____________ (use Excel Speadsheet on bench top HPLC computer) Acceptable range of radioactivity _____________to______________mCi. Measured radioactivity ____________ mCi on _____________(remember to push Co-57 button on dose calibrator; Bioscan Medical System) Does measured radioactivity fall within acceptable range (with 5% of expected value)? Yes or No (Circle one) Chemist __________________________Initials__________________ Batch # FAY-____________ Document 3. [18F]Fallypride for Injection: Quality Control Record Date_____________ Date_____________ Page 4 of 4








![[18F]NaF - revista farmacia](http://s3.studylib.net/store/data/008378966_1-99717a72f6f6a568596ed2f8f5821ecb-300x300.png)