Measuring Small Volumes and Mass
advertisement

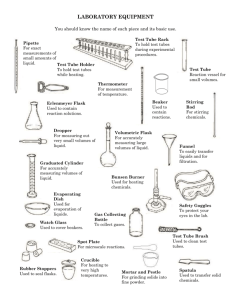
Measuring Small Volumes and Mass EXPERIMENT 1 Materials Lab coat Lab notebook Lab handout Micropipette, P-10 with tips Micropipette, P-20 with tips Micropipette, P-200 with tips Micropipette, P-1000 with tips Microcentrifuge Gloves dH2O Eppendorf Tubes Tube racks Permanent marker Balance Weigh papers Beaker for used tips BACKGROUND READING Very tiny amounts of chemicals and biological reagents are used in many biotechnology experiments. To measure these minute volumes, technicians use micropipettes that measure microliter (μL) amounts. Review the following metric conversions: Liter Milliliters (ml)…10-3 of a liter---0.001 of a liter! -6 of a liter---0.000001 of a liter! This activity introduces micropipetting technique. As with all fine motor skills, learning how to use a micropipette takes practice and determination. You must be able to measure these very tiny volumes with accuracy and precision. Operate the micropipette slowly and carefully. Picking and Setting the Micropipette 1. Check that you have the correct micropipette for the job. Most labs have three sizes of micropipettes: a P-10 (for 0.5 to 10 μL), a P-100 (for 10 to 100 μL), and a P-1000 (for 100 to 1000 μL). Note: Some laboratories have a P-20 (for 2 to 20 μL) or a P-200 (for 20 to 200 μL). 2. Dial the desired volume. Understand how to read the scale. Hint: By knowing the maximum volume of the micropipette, you can figure out what each of the digits on the readout means. 3. Push the end of the pipette into the proper-size tip. Sometimes the tips or the boxes they come in are color coded. For example, white tips are for P-10s; yellow are for P20s, P-100s, and P-200s; blue are for P-1000s. Tips are disposable and are intended for one use only. Measuring Small Volumes and Mass 1 How to Take Up a Sample with a Micropipette 4. Before picking up the micropipette, open the cap or lid of the tube from which you are taking fluid. (Skill will eventually allow you to open tubes with one hand.) 5. Hold the micropipette in one hand, at a 45º angle from vertical. In this way, contaminants from your hands or the micropipette will not fall into the tube. Hold the container in your other hand. Both should be almost at eye level. 6. Depress the plunger of the micropipette to the first stop, and hold it in this position. 7. Place the tip into the solution to be pipetted. 8. Draw fluid into the tip by slowly releasing the plunger. How to Expel a Sample from the Micropipette 9. With your other hand, open the cap or lid of the tube you are filling. 10. Hold the micropipette in one hand, at about a 45º angle from vertical. Hold the tube in your other hand. Both should be about eye level. 11. Gently touch the micropipette tip to the inside wall of the reaction tube into which you want to expel the sample. This creates a tiny surface-tension effect that helps draw the fluid out of the tip. 12. Slowly, depress the plunger of the micropipette to the first stop. Then, continue to the second stop to expel the last bit of fluid, and hold the plunger in this position. 13. Slowly, remove the pipette from the tube, keeping the plunger depressed to avoid drawing any liquid back into the tip. 14. Always change tips for each new reagent you pipette. To eject a tip, depress the ejector button on the top of the micropipette. Precautions for Using Micropipettes Set pipette volume only within the range specified for that micropipette. Do not attempt to set a volume beyond the pipette’s minimum or maximum values. When using a micropipette, first apply a tip. Failure to do this will cause liquid to enter into the nose cone. Since a micropipette works by air displacement, its internal mechanism must remain dry. Always keep a micropipette in a vertical position when there is fluid in the tip. Do not allow liquid to accidentally run back into the nose cone. Use your thumb to control the speed at which the plunger rises after taking up or ejecting fluid. Releasing the plunger too abruptly will cause leakage or bubbles that will trap air and make the measurement inaccurate. Procedures Part A. Pipetting Demonstration Part B. Get Ready 1. As specified in the Laboratory Notebook Preparation handout, setup your lab notebook for Experiment 1. Measuring Small Volumes and Mass 2 2. Put on a lab coat and gloves and wear them throughout all procedures. Part C. Practicing with a P-1000 1. Label an empty eppendorf tube as tube A. 2. To tube A, use a P-1000 to add the volumes of water shown in Table 1. Add each volume to the inside of the tube without letting the drops touch. The drops may stick because of adhesion. When all volumes have been added, bring the drops to the bottom of the tube with a quick wrist flick or gentle tapping on the lab bench. 3. Spin tube A in the microcentrifuge to pool any remaining volume in the bottom of the eppendorf tube. The instructors will show you how to use the microcentrifuge. 4. Check the accuracy of your technique with the P-1000. Set the micropipette to 810 μL and withdraw the contents of tube A. The contents should just fill the tip, with no air space at the bottom of the tip, and no leftover fluid in the tube. 5. Record the results in your lab notebook. Table 1. Volumes of water to add for P-1000 practice. Reaction Volume I Volume II Tube A 253 μL 557 μL Part D. Practicing with a P-200 1. Label an empty eppendorf tube as tube B. 2. To tube B, use a P-200 to add the volumes of water shown in Table 2. Add each volume to the inside of the tube without letting the drops touch. The drops may stick because of adhesion. When all volumes have been added, bring the drops to the bottom of the tube with a quick wrist flick or gentle tapping on the lab bench. 3. Spin tube B in the microcentrifuge to pool any remaining volume in the bottom of the eppendorf tube. 4. Check the accuracy of your technique with the P-200. Set the micropipette to 98.3 μL and withdraw the contents of tube B. The contents should just fill the tip, with no air space at the bottom of the tip, and no leftover fluid in the tube. 5. Record the results in your lab notebook. Table 2. Volumes of water to add for P-200 practice. Reaction Volume I Volume II Tube B 22.3 μL 31.6 μL Volume III 44.4 μL Part E. Practicing with a P-20 and P-10 1. Label an empty eppendorf tube as tube C. 2. To tube C, use a P-20 to add the volume I of water shown in Table 2. To tube C, use a P10 to add volumes II and III of water shown in Table 3. Add each volume to the inside of the tube without letting the drops touch. The drops may stick because of adhesion. Measuring Small Volumes and Mass 3 When all volumes have been added, bring the drops to the bottom of the tube with a quick wrist flick or gentle tapping on the lab bench. 3. Spin tube C in the microcentrifuge to pool any remaining volume in the bottom of the eppendorf tube. 4. Check the accuracy of your technique with the P-20. Set the micropipette to 12.0 μL and withdraw the contents of tube C. The contents should just fill the tip, with no air space at the bottom of the tip, and no leftover fluid in the tube. 5. Record your results in your lab notebook. Table 3. Volumes of water to add for P-20 and P-10 practice. Reaction Volume I Volume II Tube C 6.5 μL Volume III 4.3 μL 2.2 μL Part F. Checking the Accuracy of Your Micropipetting Using a Balance A balance can be used to determine if a micropipette is measuring within an acceptable range. Since 1 mL of water weighs 1.0 g, you can estimate the expected mass for any volume of water. For example: 1.5 mL of water should weigh 1.5 g. 0.25 mL of water should weigh 0.25 g. 150 μL (= 0.15 mL) of water should weigh 0.15 g. Water dispensed by a micropipette can be weighed on a balance. By comparing the actual observed mass to the expected mass, you can make an error determination. For any measurement, calculate the % error using the following equation: (observed mass – expected mass) x 100 = % error expected mass 1. For each micropipette, measure the specified volume of dH2O onto a piece of weigh paper on a “tared” balance. The instructors will show you how to use the balance. Make a copy of Table 3 below for your notebooks, and in Table 3 record the mass of the volume of water measured and calculate the % error. Table 3. Pipetting precision: mass versus volume. Micropipe Volume Volume Expected Observed tte (μL) (mL) Mass (g) Mass (g) % Error Acceptable Error (%) P-1000 257.0 3 P-200 53.0 5 P-20 20.0 5 Measuring Small Volumes and Mass 4 Part F. Finishing Up 1. Take a look at your pipetting results today, and in your lab notebook, record any final thoughts you have on your micropipetting technique. Measuring Small Volumes and Mass 5