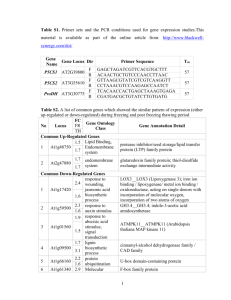
supplementary file ii : list and description of transcription factors
advertisement

SUPPLEMENTARY FILE 2 : LIST AND DESCRIPTION OF TRANSCRIPTION FACTORS PREDICTED TO BIND TO IDENTIFIED FAMILY MOTIFS PRESENT IN RICE LEA GENES. Transciption factor Function ERFLP1 , ATERF7, ERF-1, ERF-2, ERF-3, ERF-4 , ERF-5 The Ethylene-responsive factors (ERFs) are transcription factors that are unique to higher plants (Guo & Ecker, 2004). They are induced by biotic and abiotic stresses and bind GCC boxes that are present in the promoters of many PR genes (Wu et al., 2002) and have been shown to be activators and repressors of GCC box–mediated gene expression in Arabidopsis (Fujimoto et al., 2000). DREB1A, CBF1 , CBF2, CBF17 , CBF5 The DREB1 (dehydration responsive element binding)/CBF (C-repeat (CRT)-binding factor) and DREB2 transcription factors are a subclass of ERF proteins whose expression is rapidly induced by cold and dehydration respectively (Gilmour et al., 1998) (Medina et al., 1999). The DREB1/CBF TF bind to DRE and CRT ciselements and activate expression of cold responsive genes (Dubouzet et al., 2003). EmBP-1a Wheat EmBP-1 is a basic leucine zipper (bZIP) protein which has been implicated in the mechanisms of abscisic acid (ABA)-mediated expression through its ability to bind specifically to the ABA response element (ABRE) from the wheat Em (LEA) gene (Guiltinan et al., 1990). DPBF-1 , DPBF-2 The DPBF1 and 2 (Dc3 Promoter-Binding Factors) are basic leucine zipper binding proteins that were isolated from a immature seed cDNA library and identified in a screen as factors that bind to functional cis-regulatory elements in the Dc3 (carrot LEA class gene) promoter (Kim et al., 1997). They were determined to bind to ABAresponsive and embryo specification elements in the Dc3 promoter and the expression of AtDPBF-1 was found to be induced by exogenous ABA in Arabidopsis seedlings (Kim et al., 2002). TINY2 may be a new member of the AP2/EREBP transcription factor family involved in activation of down-stream genes in response to environmental stress. In Arabidopsis transcription of TINY2 was rapidly induced in response to abscisic acid, cold, drought, mechanical wounding and high salinity (Wei et al., 2005). In Gel retardation assay TINY2 was able to form a specific complex with the previously characterized DRE element. Pti4 is a tomato transcription factor that belongs to the ERF family of proteins and binds to GCC box cis-elements that are present in the promoters of many PR genes (Wu et al., 2002). In tomato, Pti4 specifically interacts with the Pto resistance gene, which encodes a Ser/Thr protein kinase (Martin et al., 1993). The Pto kinase phosphorylates Pti4 in vitro, and this phosphorylation enhances the GCC box binding activity of Pti4 (Gu et al., 2000) and expression of Pti4 in Arabidopsis resulted in induction of PR gene expression (Gu et al., 2002). TINY2 Pti4 DREBLP1 In hot pepper, Ca-DREBLP1 was rapidly induced by dehydration and high salinity. The structural features of Ca-DREBLP1 resemble those of the DREB1-type proteins of Arabidopsis thaliana and rice plants, however, its induction patterns are reminiscent of the DREB2-type proteins, suggesting that Ca-DREBLP1 is a unique class DREB subfamily in hot pepper (Hong & Kim, 2005). DBF1 , DBF2 The maize dehydration-responsive element (DRE)-binding factors, DBF1 and 2, are members of the AP2/EREBP transcription factor family. DBF1 is induced during maize embryogenesis and in seedlings after desiccation, salt and ABA treatments. DBF1 functions as an activator of DRE2-dependent transcription of rab17 promoter by ABA, whereas DBF2 overexpression had a repression action downregulating basal and ABA-induced promoter activity. ABA was thus proposed to play a role in the regulation of DBF activity possibly through an ABA-dependent pathway for the regulation of genes through the C-repeat/DRE element (Saleh et al., 2006) (Kizis & Pages, 2002). TSI1 The tobacco stress-induced gene 1 (Tsi1) has been implicated as a positive transacting factor in the regulation of gene expression in response to biotic and abiotic stresses (Park et al., 2001). Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Tsi1 bound specifically to GCC and the DRE/CRT sequences, with greater affinity to the former. DOF1 The DOF (DNA binding with One Finger) are plant specific DNA binding proteins that contain a highly conserved DOF domain with a single zinc finger that is involved in DNA-binding and protein-protein interactions (Umemura et al., 2004; Yanagisawa et al., 2004). DOF1 was originally thought to be involved in light dependent activation of transcription from the C4 phosphoenolpyruvate carboxylase (PEPC) promoter (Yanagisawa and Shenn, 1998) and induction of genes involved in carbon metabolism and nitrogen fixation (Yanagisawa, 2004) however, the role of DOF1 in the control of photosynthetic gene expression has recently been questioned (Cavalar et al., 2006). The prolamine-box binding factors (PBFs) are endosperm-specific DOF transcription factors that were isolated from maize (Vicente-Carbajosa et al., 1997). The prolamine-box is one element of the biparate endosperm box that is involved in conferring endosperm specificity in cereals (Wu et al., 2000). In barley, transcripts of the bPBF TF are present during endosperm development and bPBF proteins have been shown to be activators of reserve protein encoding genes during seed development (Mena et al., 2002). A transient expression assay system determined that barley PBF could increase transcription of the rice glutelin gene, Gt1 in developing rice endosperm cells (Hwang et al., 2004). PBF GAMYB GAMYB is a MYB class TF and has been shown to activate gene expression during endosperm development through binding to the 5’- AACAA-3’ motif in native Hor2 and Itr1 promoters (Diaz et al., 2002). The 5’- AACAAC-3’ motif has been determined to be the third important motif in regulating endosperm specificity and is present in the rice glutelin gene promoters and is recognized by TFs of the MYB class (Suzuki et al., 1998). In barley, transcripts of GAMYB were detected early in the developing seed and were determined to accumulate in the aleurone layer, starchy endosperm, nuclear projection and vascular tissue, but also in the immature embryo. Evidence indicates that GAMYB is an important TF in the combinatorial regulation of genes specifically expressed in the developing endosperm (Diaz et al., 2002). MCB1 , MCB2 The MCB1 gene encodes a R1MYB protein that binds to the 5'TATCCAC-3' (GATA core) box in vitro and is a transcriptional repressor of a GA-induced amylase (Amy6.4) promoter in bombarded aleurone layers (Rubio-Somoza et al., 2006). In barley, MCB1 repressed transcription of the Amy6.4 promoter in GA-treated aleurone layers and reversed the GAMYB-mediated activation of this amylase promoter. In contrast, during endosperm maturation HvMCB1 acted as a transcription activator of the seed-specific Itr1 gene promoter through binding to a 5'-GATAAGATA-3' box (Rubio-Somoza et al., 2006). The calmodulin-binding transcription activators (CAMTAs) comprise a conserved family of transcription factors in a wide range of multicellular eukaryotes (Bouche et al., 2002). In plants, CAMTAs were identified in a screening of cDNA expression libraries of drought stressed leaves using a recombinant calmodulin probe (Bouche et al., 2002). The proteins were determined to contain a transcription activation domain CAMTA1 CAMTA3 and two DNA-binding domains, designated the CG-1 domain and the transcription factor immunoglobulin (TIG)-like domain, ankyrin repeats, and a number of calmodulin-binding motifs (Lander et al., 2001) (Bouche et al., 2002). In Arabidopsis six CAMTA genes (AtCAMTA1-AtCAMTA6) that are also referred to as AtSR1–6 (Arabidopsis thaliana signal-responsive genes) have been identified and these genes have been determined to be rapidly and differentially expressed in response to environmental signals including UV, extreme temperatures, high salt, the hormones ethylene and ABA as well as signaling molecules such as methyl jasmonate, H2O2, and salicylic acid (Yang & Poovaiah, 2002). The CAMTAs have been determined to specifically bind to a CGCG box (Chen et al., 2005) (Bouche et al., 2002). CBT The OsCBT (Oryza sativa CaM-binding transcription factor) is a putative calmodulinbinding transcription factor that was isolated from a rice cDNA expression library using CaM:horseradish peroxidase as a probe. OsCBT shared similar structural features to Arabidopsis AtSRs/AtCAMTAs factors and was determined to preferentially bind to 5’-TWCG(C/T)GTKKKKTKCG-3’ (W = A or C and K = T or G) DNA sequences and also bind to an oligonucleotide probe containing CGCG motifs that was selected from the stress inducible rice Phenylalanine ammonia-lyase (PAL,ZB8) gene promoter supporting that OsCBT DB binds DNA (Choi et al., 2005). (VOZ1)2 , (VOZ2)2 AtVOZ1 and AtVOZ2, are novel transcription factors that bind to a 38-bp cis-acting region of the A. thaliana V-PPase gene (AVP1), which is a vacuolar proton pump that is coupled with PPi hydrolysis (Maeshima, 2001). The transcription of AVP1 is altered in response to various environmental conditions and developmental stages (Maeshima, 2001). ANT The AINTEGUMENTA (ANT), gene promotes initiation and growth of lateral organ primordial (Nole-Wilson & Krizek, 2006). Results indicate that ANT acts in combination with the YABBY gene FILAMENTOUS FLOWER (FIL) to promote organ polarity by up-regulating the expression of the adaxial-specifying HD-ZIP gene PHABULOSA and the floral homeotic gene APETALA3. JAMYC2 The JAMYC2 gene encodes a MYC regulatory bHLH-Leucine zipper DNA-binding protein that specifically binds Jasmonic acid (JA) responsive elements present in wound-inducable tomato genes including the proteinase inhibitor II (pin2) and leucine aminopeptidase (LAP) genes. JAMYC2 specifically binds to a T/G-box AACGTG motif in the tomato LAP gene promoter which is important for JA-mediated defence responses. Knockouts of the Arabidopsis homolog gene AtMYC2 resulted in JAinsensitivity and the JAMYC/AtMYC2 transcription factors are believed to pay a key role in JA-induced defense gene activation (Boter et al., 2004). ARR10 The Arabidopsis response regulators (ARR) comprise a family of 22 response regulators that are classified into type A and B subtypes. The type B ARRs are putative transcription factors and include ARR10. The ARRs are thought to be involved in a multistep phosphorelay signaling pathway in response to cytokinin signaling (Suzuki et al., 2001). A model of action for cytokinin signaling has been proposed that relays the cytokinin signal from the membrane to the nucleus via a multi-step phosphorelay that involves cytokinin receptors, AHP (Arabidopsis Hiscontaining phosphotransfer factor) and type-B ARRs which induce transcription of the type-A genes which may mediate downstream responses to cytokinins (Hwang & Sheen, 2001) (Kakimoto, 2003; Hutchison & Kieber, 2002). It has been proposed that ARR10,-12, and -1 together redundantly play pivotal roles in the AHK-dependent phosphorelay signaling in response to cytokinin in roots (Yokoyama et al., 2007). GT-1,-2 GT-1 and GT-2 are are trihelix DNA-binding factors that bind to GT elements found in the promoters of many light responsive plant genes and have been shown to be involved in light-responsive transcription in vivo (Schindler and Cashmore, 1990). Gain-of-function experiments have confirmed a critical role for GT-1 cis-elements in mediating light-responsive and tissue-specific gene expression (Hiratsuka et al., 1994) . The GT-2 TF, that was first cloned from rice, was found to bind to a triplet of positively acting GT-boxes (GT-1, -2, -3 bx) that are present in the rice phytochrome A (PHYA) gene promoter, having greatest affinity for GT2-bx and GT3-bx motifs (Dehesh et al., 1990). Studies with recombinant GT-2 indicate that it functions as a transcriptional activator in vivo(Ni et al., 1996). GT-3a The GT-3a factor is a relatively recently identified GT factor belonging to a subgroup that cannot bind to GT-1 or -2 binding sites but can bind to cab2 and rbcS-1A gene promoters via the 5’-GTTAC sequence. It has been determined to be predominantly expressed in floral buds and roots and form homo- or heterodimers (Ayadi et al., 2004). PF1 PF1 is a small nuclear protein of the high mobility group (HMG) I/Y type that is involved in the regulation of phytochrome A (PHYA) transcription. The PF1 protein contains 4 repeats of the ‘A/T-hook’ motif that mediates binding to the narrow groove of A/T-rich DNA (Reeves & Nissen, 1990). It binds to a cognate A/T-rich ciselement termed PE1 that is present in the PHYA promoter and positively regulates its transcription (Martinez-Garcia & Quail, 1999). The phytochrome family consists of red- and far-red-light absorbing sensory photoreceptors that regulate adaptational changes in gene expression in response to environmental light signals in plants (Tepperman et al., 2001). PHYA is exclusively responsible for seedling responsiveness to continuous far-red light (Deng & ail, 1999; Whitelam & Devon, 1997) and may regulate seedling photomorphogenesis (Tepperman et al., 2001). TBP2 The TATA binding protein (TBP) is a subunit of the TFllD transcription initiation factor that interacts with the TATA box sequence in the promoter of most eukaryotic nuclear protein-encoding genes and performs an early DNA recognition function for RNA polymerase II (Vogel et al., 1993). TBP additionally serves as a critical subunit in the initiation complexes for RNA polymerases I and III (reviewed in (Green, 1992)). TBP and TFIIB play crucial roles in transcription of class II genes (Pan et al., 2000). In yeast, animals, and insects (see (Peterson et al., 1990)) only one TBP encoding gene has been identified while in Arabidopsis and maize two TBP genes encoding distinct TBP isoforms have been isolated (Gasch et al., 1990) (Haass & Feix, 1992). In some maize tissue, quantitative differences in transcript accumulation was observed between TBPl and TBP2 and it was suggested that they could differ in their DNA binding specificity and/or in specific interactions with other transcription proteins (Vogel et al., 1993). DBP1 DBP1 is a novel DNA-binding protein from tobacco plants with protein phosphatase activity, which binds in a sequence-specific manner to a cis- acting element of a defense-related gene and participates in its transcriptional regulation (Carrasco et al., 2003). GCBP-1 , GCBP-1 binds to the minimum consensus sequence 5'-GC(G/C)CC-3' which is similar to a part of the binding site of the human transcription factor Sp1. Maize GCBP-1 and human Sp1 were determined to have similar recognition properties. Studies implicate a direct role for GCBP-1 in the hypoxic activation of Alcohol dehydrogenase-1 (Adhl) gene expression (Olive et al., 1991). Sp1 The human transcription factor Sp1 also binds to the ARE element and has similar DNA recognition sites as GCBP-1 (Olive et al., 1991). Sp1 is essential for early embryonic development in mouse (Martin & PazAres, 1997). References Ayadi M, Delaporte V, Li YF, Zhou DX. 2004. Analysis of GT-3a identifies a distinct subgroup of trihelix DNA-binding transcription factors in Arabidopsis 555. FEBS Lett 562: 147-154. Boter M, Ruiz-Rivero O, Abdeen A, Prat S. 2004. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev. 18: 1577-1591. Bouche N, Scharlat A, Snedden W, Bouchez D, Fromm H. 2002. A novel family of calmodulin-binding transcription activators in multicellular organisms. J.Biol Chem. 277: 21851-21861. Carrasco JL, Ancillo G, Mayda E, Vera P. 2003. A novel transcription factor involved in plant defense endowed with protein phosphatase activity. EMBO J. 22: 3376-3384. Chen X, Yu T, Xiong J, Zhang Y, Hua Y, Li Y, Zhu Y. 2005. Molecular cloning and expression analysis of rice phosphoribulokinase gene that is regulated by environmental stresses. Molecular Biology Reports 31: 249-255. Choi MS, Kim MC, Yoo JH, Moon BC, Koo SC, Park BO, Lee JH, Koo YD, Han HJ, Lee SY, Chung WS, Lim CO, Cho MJ. 2005. Isolation of a calmodulin-binding transcription factor from rice (Oryza sativa L.) 1. J Biol Chem 280: 40820-40831. Dehesh K, Bruce WB, Quail PH. 1990. A trans-acting factor that binds to a GT-motif in a phytochrome gene promoter 1. Science 250: 1397-1399. Deng X, ail PH. 1999. Signalling in light-controlled. development. 579. Semin.Cell Dev.Biol. 10, 121-129. Diaz I, Vicente-Carbajosa J, Abraham Z, Martinez M, Isabel-La Moneda I, Carbonero P. 2002. The GAMYB protein from barley interacts with the DOF transcription factor BPBF and activates endosperm-specific genes during seed development. Plant J. 29: 453-464. Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2003. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 33: 751-763. Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M. 2000. Arabidopsis ethyleneresponsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 12: 393-404. Gasch A, Hoffmann A, Horikoshi M, Roeder RG, Chua NH. 1990. Arabidopsis thaliana contains two genes for TFIID 562. Nature 346: 390-394. Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF. 1998. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 16: 433-442. Green MR. 1992. Gene regulation. Transcriptional transgressions. Nature 357: 364-365. Gu YQ, Wildermuth MC, Chakravarthy S, Loh YT, Yang C, He X, Han Y, Martin GB. 2002. Tomato transcription factors pti4, pti5, and pti6 activate defense responses when expressed in Arabidopsis 552. Plant Cell 14: 817-831. Gu YQ, Yang C, Thara VK, Zhou J, Martin GB. 2000. Pti4 is induced by ethylene and salicylic acid, and its product is phosphorylated by the Pto kinase 553. Plant Cell 12: 771-786. Guiltinan MJ, Marcotte WR, Jr., Quatrano RS. 1990. A plant leucine zipper protein that recognizes an abscisic acid response element 1. Science 250: 267-271. Guo H, Ecker JR. 2004. The ethylene signaling pathway: new insights 551. Curr Opin Plant Biol 7: 40-49. Haass MM, Feix G. 1992. Two different cDNAs encoding TFIID proteins of maize 1. FEBS Lett 301: 294-298. Hiratsuka K, Wu X, Fukuzawa H, Chua NH. 1994. Molecular dissection of GT-1 from Arabidopsis 1. Plant Cell 6: 1805-1813. Hong JP, Kim WT. 2005. Isolation and functional characterization of the Ca-DREBLP1 gene encoding a dehydration-responsive element binding-factor-like protein 1 in hot pepper (Capsicum annuum L. cv. Pukang) 1. Planta 220: 875-888. Hutchison CE, Kieber JJ. 2002. Cytokinin signaling in Arabidopsis 1. Plant Cell 14 Suppl: S47-S59. Hwang I, Sheen J. 2001. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413: 383-389. Hwang YS, Ciceri P, Parsons RL, Moose SP, Schmidt RJ, Huang N. 2004. The maize O2 and PBF proteins act additively to promote transcription from storage protein gene promoters in rice endosperm cells. Plant Cell Physiol 45: 1509-1518. Kakimoto T. 2003. Perception and signal transduction of cytokinins. Annu.Rev Plant Biol 54: 605-627. Kim SY, Chung HJ, Thomas TL. 1997. Isolation of a novel class of bZIP transcription factors that interact with ABA-responsive and embryo-specification elements in the Dc3 promoter using a modified yeast one-hybrid system. Plant J. 11: 1237-1251. Kim SY, Ma J, Perret P, Li Z, Thomas TL. 2002. Arabidopsis ABI5 subfamily members have distinct DNA-binding and transcriptional activities. Plant Physiol 130: 688-697. Kizis D, Pages M. 2002. Maize DRE-binding proteins DBF1 and DBF2 are involved in rab17 regulation through the drought-responsive element in an ABA-dependent pathway. Plant J. 30: 679-689. Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, StangeThomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la BM, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ. 2001. Initial sequencing and analysis of the human genome. Nature 409: 860-921. Maeshima M. 2001. TONOPLAST TRANSPORTERS: Organization and Function 1. Annu Rev Plant Physiol Plant Mol Biol 52: 469-497. Martin C, PazAres J. 1997. MYB transcription factors in plants. Trends in Genetics 13: 67-73. Martin GB, Brommonschenkel SH, Chunwongse J, Frary A, Ganal MW, Spivey R, Wu T, Earle ED, Tanksley SD. 1993. Map-based cloning of a protein kinase gene conferring disease resistance in tomato 597. Science 262: 1432-1436. Martinez-Garcia JF, Quail PH. 1999. The HMG-I/Y protein PF1 stimulates binding of the transcriptional activator GT-2 to the PHYA gene promoter. Plant J. 18: 173-183. Medina J, Bargues M, Terol J, Perez-Alonso M, Salinas J. 1999. The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression Is regulated by low temperature but not by abscisic acid or dehydration. Plant Physiol 119: 463-470. Mena M, Cejudo FJ, Isabel-Lamoneda I, Carbonero P. 2002. A role for the DOF transcription factor BPBF in the regulation of gibberellin-responsive genes in barley aleurone. Plant Physiol 130: 111-119. Ni M, Dehesh K, Tepperman JM, Quail PH. 1996. GT-2: in vivo transcriptional activation activity and definition of novel twin DNA binding domains with reciprocal target sequence selectivity 1. Plant Cell 8: 1041-1059. Nole-Wilson S, Krizek BA. 2006. AINTEGUMENTA contributes to organ polarity and regulates growth of lateral organs in combination with YABBY genes 1. Plant Physiol 141: 977-987. Olive MR, Peacock WJ, Dennis ES. 1991. The anaerobic responsive element contains two GC-rich sequences essential for binding a nuclear protein and hypoxic activation of the maize Adh1 promoter. Nucleic Acids Res 19: 7053-7060. Pan S, Czarnecka-Verner E, Gurley WB. 2000. Role of the TATA binding proteintranscription factor IIB interaction in supporting basal and activated transcription in plant cells 560. Plant Cell 12: 125-136. Park JM, Park CJ, Lee SB, Ham BK, Shin R, Paek KH. 2001. Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 13: 1035-1046. Peterson MG, Tanese N, Pugh BF, Tjian R. 1990. Functional domains and upstream activation properties of cloned human TATA binding protein. Science 248: 1625-1630. Reeves R, Nissen MS. 1990. The A.T-DNA-binding domain of mammalian high mobility group I chromosomal proteins. A novel peptide motif for recognizing DNA structure. J.Biol Chem. 265: 8573-8582. Rubio-Somoza I, Martinez M, Diaz I, Carbonero P. 2006. HvMCB1, a R1MYB transcription factor from barley with antagonistic regulatory functions during seed development and germination. Plant J. 45: 17-30. Saleh A, Lumbreras V, Lopez C, Dominguez-Puigjaner E, Kizis D, Pages M. 2006. Maize DBF1-interactor protein 1 containing an R3H domain is a potential regulator of DBF1 activity in stress responses. Plant J. 46: 747-757. Suzuki A, Wu CY, Washida H, Takaiwa F. 1998. Rice MYB protein OSMYB5 specifically binds to the AACA motif conserved among promoters of genes for storage protein glutelin. Plant Cell Physiol 39: 555-559. Suzuki T, Sakurai K, Ueguchi C, Mizuno T. 2001. Two types of putative nuclear factors that physically interact with histidine-containing phosphotransfer (Hpt) domains, signaling mediators in His-to-Asp phosphorelay, in Arabidopsis thaliana 1. Plant Cell Physiol 42: 37-45. Tepperman JM, Zhu T, Chang HS, Wang X, Quail PH. 2001. Multiple transcriptionfactor genes are early targets of phytochrome A signaling. Proc.Natl.Acad.Sci U.S.A 98: 9437-9442. Vicente-Carbajosa J, Moose SP, Parsons RL, Schmidt RJ. 1997. A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque2. Proc.Natl.Acad.Sci U.S.A 94: 7685-7690. Vogel JM, Roth B, Cigan M, Freeling M. 1993. Expression of the two maize TATA binding protein genes and function of the encoded TBP proteins by complementation in yeast 558. Plant Cell 5: 1627-1638. Wei G, Pan Y, Lei J, Zhu YX. 2005. Molecular cloning, phylogenetic analysis, expressional profiling and in vitro studies of TINY2 from Arabidopsis thaliana. J.Biochem Mol Biol 38: 440-446. Whitelam GC, Devon K. 1997. Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ. 20, 752-758. Wu C, Washida H, Onodera Y, Harada K, Takaiwa F. 2000. Quantitative nature of the Prolamin-box, ACGT and AACA motifs in a rice glutelin gene promoter: minimal ciselement requirements for endosperm-specific gene expression. Plant J. 23: 415-421. Wu K, Tian L, Hollingworth J, Brown DC, Miki B. 2002. Functional analysis of tomato Pti4 in Arabidopsis 554. Plant Physiol 128: 30-37. Yang T, Poovaiah BW. 2002. A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J.Biol Chem. 277: 4504945058. Yokoyama A, Yamashino T, Amano Y, Tajima Y, Imamura A, Sakakibara H, Mizuno T. 2007. Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant Cell Physiol 48: 84-96.