Langmuir & Freundlich Isotherms: Phenol Adsorption on GAC
advertisement

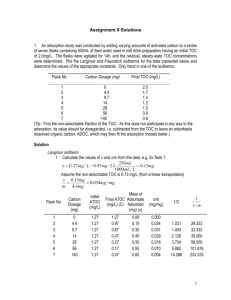
Derivation of Langmuir and Freundlich Isotherms by Using HPLC Abstract: Different amounts of granulated activated carbon were combined with a phenol solution in eight bottles. After the phenol was allowed to adsorb onto the surface of the activated carbon the remaining phenol in solution was measured by HPLC. Langmuir and Freundlich isotherms were then derived from our data. The Langmuir isotherm is a better representation for lower concentrations and the Freundlich isotherm is a better representation at higher concentrations. Introduction: The fate of many chemical pollutants in the environment depends on whether or not it is capable of adsorbing to particles. Sorption isotherms are used to describe equilibrium between sorbed and desorbed phases. There are many different sorption isotherms each with a different shape (1). The two isotherms used in this lab were the Langmuir and Freundlich isotherms. The Freundlich isotherm is described by the following equation: Cis K iF Ciwni The Langmuir isotherm is described by this equation: K C Cis max iL iw 1 K iL Ciw In this lab granular activated carbon was put into a phenol solution to allow the adsorption of phenol on its surface. The remaining phenol in the solution was then measured by high performance liquid chromatography (HPLC). HPLC uses a liquid phase that is forced through a column at high pressure (2). The purpose of this experiment was to determine whether the Langmuir isotherm or Freundlich isotherm is a better fit for the adsorption of phenol to granular activated carbon. Procedure: Standard Preparation: Standards were prepared from a 100mg/L buffered phenol solution (190 mg/L KH2PO4 and 100 mg/L K2HPO4). The concentrations of the standards were as follows: Std (mg/L) 5.08 10.16 25.4 50.8 101.6 Sample Preparation: The samples were prepared by adding differing amounts of granular activated carbon (GAC) and the phenol solution in eight different bottles. The bottles preparation is summarized in the following table: Bottle # Bottle (g) 1 2 3 4 5 6 7 8 62.706 62.797 63.175 62.248 62.903 62.970 63.013 62.731 GAC (g) Phenol Vol (ml) 0.0910 0.0770 0.0570 0.0430 0.0290 0.0140 0.0060 0.0000 48.16 48.21 48.88 47.50 50.49 49.92 48.02 47.34 Bottle+GAC Bottle+GAC+Phenol (g) (g) 62.797 62.874 63.232 62.291 62.932 62.984 63.019 62.731 110.959 111.082 112.115 109.794 113.421 112.903 111.034 110.071 Analysis: After the samples were left in a shaker for seven days the phenol solution was separated from the GAC and analyzed using HPLC. The Freundlich and Langmuir Isotherms were then calculated by using the following formulas: Langmuir: K C 1 1 1 1 Cis max iL iw 1 K iL Ciw Cis Cis,max K iL Ciw Cis,max ni Freundlich: Cis K iF Ciw log Cis n log Ciw log K iF Results: Figure 1: Standard Curve Phenol Standard Curve y = 32.65018x - 1.13831 R2 = 0.99999 3500 3000 Area 2500 2000 1500 1000 500 0 0 20 40 60 80 100 120 Concentration (m g/L) This standard curve was used to calculate phenol concentration in the samples. Table 1: Calculated Values for Cw and Cs Bottle # Cw (mg/L) Cs (mg/g) log Cw log Cs 1/Cw 1/Cs 1 2 3 4 5 6 7 1.5 2.0 5.1 6.6 22.9 51.6 80.2 53.0 62.4 82.8 105.0 137.0 178.2 170.9 0.173093 0.297276 0.706324 0.818834 1.359692 1.712778 1.904424 1.724142 1.794963 1.917876 2.021025 2.136815 2.250975 2.232701 0.671286 0.504341 0.196642 0.151763 0.043683 0.019374 0.012462 0.018874 0.016034 0.012082 0.009527 0.007298 0.005611 0.005852 Figure 2: Sorption Curve Cs vs. Cw Cs (mg/g) 200 150 100 50 0 0 20 40 60 80 100 Cw (mg/L) Figure 3: Langmuir Isotherm Langmuir Isotherm y = 0.019516x + 0.006294 R2 = 0.963782 0.025 0.02 1/Cs 0.015 0.01 0.005 0 0 0.2 0.4 1/Cw 1 1 1 1 Cis Cis,max K iL Ciw Cis,max 0.6 0.8 Figure 4: Freundlich Isotherm log Cs Freundlich Isotherm 2.4 2.3 2.2 2.1 2 1.9 1.8 1.7 1.6 1.5 y = 0.3021x + 1.7103 R2 = 0.9649 0 0.5 1 1.5 2 log Cw log Cis n log Ciw log K iF Table 2: Isotherm Values Freundlich n log KF KF 0.3021 1.7103 51.32158 Langmuir Cs max KL 158.8815 0.32201 These values were obtained from the linearization of each isotherm. Table 3: Comparison of Langmuir and Freundlich Isotherm Langmuir vs. Freundlich Isotherms 250.0 Cs (mg/g) 200.0 Cs (mg/g) 150.0 Freundlich Langmuir 100.0 50.0 0.0 0.0 20.0 40.0 60.0 Cw (mg/L) 80.0 100.0 Conclusions: The Langmuir isotherm was a better adsorption model at lower Cw concentrations and the Freundlich was the better model at higher Cw concentrations. This gives reason to believe that the adsorption of phenol to GAC is most likely a mixed isotherm that combines both the Freundlich and Langmuir isotherms. A better representation of which isotherm is the better fit may be achievable by testing a larger amount of samples with differing amounts of phenol and GAC. This would give us more points to plot on a graph which would hopefully allow us to more accurately plot the isotherms. There was little error in this study. All of the values we calculated make sense. Also the sample we used that did not have any GAC did not show any significant detection of phenol adsorption so blank subtraction was not necessary. We achieved our purpose in this experiment by showing that both the Langmuir and Freundlich isotherms are good representations of adsorption at different concentration. The only part of this study that could be improved would be an increased number of samples. Acknowledgements: I’d like to thank Dr. Lisa Rodenburg and Jia Guo for their assistance with this lab. References: 1. Rodenburg, L. Sorption to organic matter. Power Point Presentation. 2008. 2. Rodenburg, L. Introduction to Chromatography. Lecture. 2008.