EMI4_270_sm_supp_info
advertisement

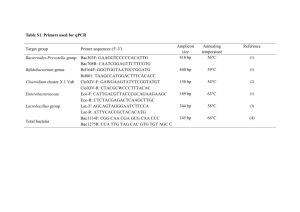
Supplementary Information 1 Site description, soil collection and soil treatments Landfill soil was collected from a local landfill site in Ufton, UK (latitude 52° 15' 0 N; longitude 1° 25' 60 W) in April 2008. The vegetation, predominantly grass above the cover soil, was cleared before collecting soil to a depth of 30 cm. The moisture content of the soil at the time of sampling was measured by oven drying soil subsamples (after removing stones) at 80oC until constant weight (26.4% ± 1.3). The soil was stored at 40C until use. Nucleic acid extraction, purification and cDNA synthesis Total nucleic acids were extracted using 0.5 g of soil sub-samples from time 0, I and II by employing a modified bead beating protocol described by Chen et al. (2007). Nucleic acid extractions for each soil treatments were done in triplicate (single extraction from two 13C- and one 12C-CH4 soil incubations in serum bottles). RNA samples were confirmed to be DNA-free by lack of amplification of 16S rRNA genes with the universal bacterial primer set 27f/907r (Lane, 1991; Muyzer et al., 1993) (30 cycles of 94oC for 1 min, 60 oC for 1 min and 72 oC for 1 min with a final extension at 72 oC for 10min). Nucleic acid concentrations were quantified using a Nanodrop spectrophotometer (Nanodrop). SuperScriptTM II Reverse Transcriptase kit (Invitrogen) was used to synthesize cDNA from 100 ng of RNA, following the manufacturer’s instructions, using primers mb661r for pmoA (Costello and Lidstrom, 1999). cDNA generated was used as a template for PCR (35 cycles) using pmoA primers A189f/ mb661r (Costello and Lidstrom, 1999; Holmes et al., 1999) at an annealing temperature of 55oC to confirm the reverse transcription reaction. Control reactions were prepared as described above, but without reverse transcriptase to check for DNA contamination in RNA samples. Replicate RNA samples (two 13C-CH4 soil incubations in serum bottles) from each soil treatments were used to synthesize cDNA representing pmoA genes. These samples were subsequently pooled and subjected to pmoA microarray analysis. DNA-Stable isotope probing DNA extracted from time II was used for DNA-SIP analysis. One microgram of DNA extracted at time II (pooled DNA from two 13C-CH4 soil incubations) was used for density gradient ultra-centrifugation followed by gradient fractionation, precipitation of DNA and buoyant density measurements of the fractions as described by Neufeld et al. (2007). pmoA microarray analysis PCR amplification of pmoA genes from DNA and cDNA were performed with primer set A189f/T7-mb661r (Bourne et al., 2001) in duplicate reactions (from two 13C-CH4 serum bottles), which were subsequently pooled following PCR. PCR was carried out over 35 cycles at 95oC for 1 min, 55oC for 1 min, 72oC for 1 min, and a final extension step at 72oC for 10 min in a Biorad thermal cycler. Each PCR reaction contained 1× reaction buffer, 0.2 mM dNTPs, 2.5 mM MgCl2, 0.2 M of each primer (forward and reverse) (Invitrogen) and 2.5 units of Taq DNA polymerase (Fermentas). In vitro transcription, RNA purification and microarray hybridisation for DNA were performed according to Stralis-Pavese et al (2004) and for mRNA as described by Bodrossy et al (2006). After hybridization, slides were scanned using a GenePix 4000 A laser scanner (Axon) at 10 mm resolution at 532 nm wavelength (Cy3). Results were then normalized to a positive control probe (mtrof 173) (Bodrossy et al., 2003). References Bodrossy, L., Stralis-Pavese, N., Murrell, J.C., Radajewski, S., Weilharter, A., and Sessitsch, A. (2003) Development and validation of a diagnostic microbial microarray for methanotrophs. Environ Microbiol 5: 566-582. Bodrossy, L., Stralis-Pavese, N., Konrad-Koszler, M., Weilharter, A., Reichenauer, T.G., Schofer, D., and Sessitsch, A. (2006) mRNA-based parallel detection of active methanotroph populations by use of a diagnostic microarray. Appl Environ Microbiol 72: 1672-1676. Bourne, D.G., McDonald, I.R., and Murrell, J.C. (2001) Comparison of pmoA PCR primer sets as tools for investigating methanotroph diversity in three Danish soils. Appl Environ Microbiol 67: 3802-3809. Chen, Y., Dumont, M.G., Cebron, A., and Murrell, J.C. (2007) Identification of active methanotrophs in a landfill cover soil through detection of expression of 16S rRNA and functional genes. Environ Microbiol 9: 2855-2869. Costello, A.M., and Lidstrom, M.E. (1999) Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl Environ Microbiol 65: 5066-5074. Holmes, A.J., Roslev, P., McDonald, I.R., Iversen, N., Henriksen, K., and Murrell, J.C. (1999) Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake. Appl Environ Microbiol 65: 3312-3318. Lane, D.J. (1991) 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics. Stackebrandt, E., and Goodfellow, M. (eds). New York, USA: John Wiley & Sons, pp. 115–147. Muyzer, G., de Waal, E.C., and Uitterlinden, A.G. (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59: 695-700. Neufeld, J.D., Vohra, J., Dumont, M.G., Lueders, T., Manefield, M., Friedrich, M.W., and Murrell, J.C. (2007) DNA stable-isotope probing. Nat Protoc 2: 860-866. Stralis-Pavese, N., Sessitsch, A., Weilharter, A., Reichenauer, T., Riesing, J., Csontos, J. et al. (2004) Optimization of diagnostic microarray for application in analysing landfill methanotroph communities under different plant covers. Environ Microbiol 6: 347-363.