a Reimbursement Appeals Letter Template
advertisement

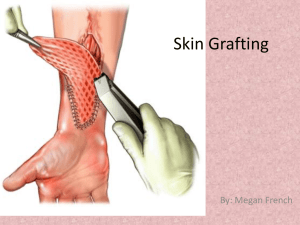
Gamma Graft Sample Appeals Letter Date: Patient Policy Holder ID/Social Security # Contract # Insurance Company Address City, State, Zip Attention: (Name) Dear _________: I am writing to request reconsideration of your decision to deny payment for the application of Gamma Graft used for the treatment of (Patient Name)’s (type of wound) on (insert date of service here), who is insured by your company. Paragraph #2: Describe the patient’s condition Document current findings/status Document chronological history (Patient name) clearly benefited from the use of GammaGraft. Describe how the patient benefited from the use of GammaGraft: (ex. Wound has healed…) Recommend considerations for use of GammaGraft The use of allograft skin obtained from human cadavers is considered to be the gold standard treatment to provide temporary wound closure when the patient’s own skin is not available. Allograft is self-adhering, renders the wound site pain free, and acts as a vapor barrier that limits fluid and protein loss from the wound while preventing infection. Use of cadaver skin has been limited to use in the hospital setting due to handling requirements. GammaGraft is human skin allograft acquired from cadaveric donors that has been irradiated to allow for storage at room temperature. GammaGraft has both the epidermal and the dermal layers of human skin which makes it more durable and effective as a vapor barrier than most wound covers, especially some artificial skins, which lack a keratinocyte layer (found in the epidermis). Its main applications are as a temporary graft for treating burns, chronic wounds, and partial and full thickness wounds. The irradiation process that GammaGraft undergoes produces two key advantages. The irradiation acts as a preservation and sterilization agent significantly reducing any risk of viral transmission of disease and allowing it to be stored at room temperature for up to two years. This is an added precaution to eliminate viruses beyond the extensive serological testing done by the tissue banks to ensure virus-free skin. In fact GammaGraft has been treated so as to greatly reduce the threat of CMV (cytomegalovirus). It is therefore ideal for use in transplant patients, HIV positive patients, and other people with compromised immune systems who could be adversely affected by CMV. It is important to draw the distinction between GammaGraft and other tissue engineered skin substitute such as Apligraf. Unlike Apligraf and the other skin substitutes which are regulated as devices by the FDA, GammaGraft is regulated by the FDA as human tissue because it is donated human skin and not an engineered product. Furthermore, the Centers for Medicare and Medicaid Services recently published their intent to establish a unique billing code for GammaGraft in recognition of the fact that the existing codes describing tissue engineered skin substitutes do not apply to GammaGraft as is the case with fresh or frozen cadaver skin as well. This new code will become available January 1, 2009 until which time it is necessary to bill for the product using the unlisted biologic code J3590. Insert the following if standard cadaver grafts are covered by the plan: Since GammaGraft is actually human cadaver skin and is regulated similarly by the FDA, it should be covered just as non-irradiated cadaver skin is under your policy. Insert the following if unsure about coverage status of cadaver grafts: The use of human allograft skin is considered to be the standard of care when there is a need for temporary wound closure. GammaGraft is human cadaver skin; but, with the additional advantages offered by the irradiation process. As such, it should be considered for coverage by (insert insurance company name). I have included some information about GammaGraft as well as the patient’s medical records for your review. I think that once you learn more about the product, you will agree that this claim should in fact be paid. I would like the opportunity to discuss this matter with you further. I will contact you on (Date) to schedule a time for us to discuss this appeal. In the meantime, if you have any questions, please contact me. Sincerely, ____, M.D. Enclosures: Summary of relevant clinical articles Product Information Patient Medical Records