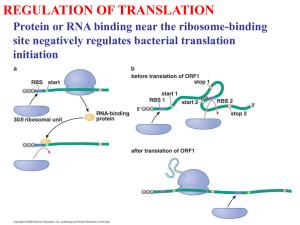
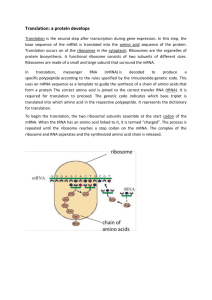
Chapter Fifteen: The Genetic Code and Translation
advertisement

Chapter Fifteen: The Genetic Code and Translation COMPREHENSION QUESTIONS 1. What is the one gene, one enzyme hypothesis? Why was this hypothesis an important advance in our understanding of genetics? The one gene, one enzyme hypothesis proposed by Beadle and Tatum states that each gene encodes a single, separate protein. Now that we know more about the nature of enzymes and genes, it has been modified to the one gene, one polypeptide hypothesis since many enzymes consist of multiple polypeptides. The original hypothesis helped establish a linear link between genes (DNA) and proteins. 2. What three different methods were used to help break the genetic code? What did each reveal and what were the advantages and disadvantages of each? Marshall Nirenberg and Johann Heinrich Matthaei used the enzyme polynucleotide kinase to create homopolymers of synthetic RNAs. Using a cell-free protein synthesizing system they were able to determine the amino acid coded by each homopolymer. By this method, the meanings for the amino acids specified by the codons UUU, AAA, CCC, and GGG were determined. The disadvantage is that the meanings for only four codons could be determined. The same system was also used to create copolymers that contained random mixtures of two nucleotides in a known ratio. Different amino acids in the protein depended on the ratio of the two nucleotides. To determine or predict the composition of the codons, the frequency of amino acids produced using the copolymer was compared with the theoretical frequencies expected for the codons. A disadvantage of this procedure is that it depended on random incorporation of the nucleotides, which did not always happen. A further problem was that the base sequence of the codon could not be determined—only the bases contained within the codon. The redundancy of the code also provided difficulties since several different codons could specify the same amino acid. To solve these problems, Nirenberg and Leder mixed ribosomes bound to short RNAs of known sequences with charged tRNAs. The mixture was passed through a nitrocellulose filter to which the tRNAs paired to ribosome-mRNA stuck. They next determined the amino acids attached to the bound tRNAs. Over 50 codons were identified by this method. The difficulty is that not all tRNAs and codons could be identified with this method. Gobind Khorana and his colleagues used a third method. They synthesized RNA molecules of known repeating sequences. Using a cell-free protein synthesizing system they produced proteins of alternating amino acids. However, this procedure could not specify which codon encodes which amino acid. 3. What are isoaccepting tRNAs? Isoaccepting tRNAs are tRNA molecules that have different anticodon sequences but accept the same amino acids. Chapter Fifteen: The Genetic Code and Translation 187 4. What is the significance of the fact that many synonymous codons differ only in the third nucleotide position? Synonymous codons code for the same amino acid, or, in other words, have the same meaning. A nucleotide at the third position of a codon pairs with a nucleotide in the first position of the anticodon. Unlike the other nucleotide positions involved in the codon-anticodon pairing, this pairing is often weak or “wobbles,” and nonstandard pairings can occur. Since many synonymous codons differ at only the third nucleotide position, it is likely that in these codons the “wobble” and nonstandard base-pairing with the anticodons will result in the correct amino acid being inserted in the protein even if a nonstandard pairing occurs. 5. Define the following terms as they apply to the genetic code: (a) Reading frame The reading frame refers to each different way that the groups of three nucleotides or codons can be read in a sequence of nucleotides. For any sequence of nucleotides, there are potentially three sets of codons that could specify the amino acid sequence of a polypeptide. (b) Overlapping code If an overlapping code is present, then a single nucleotide is included in more than one codon. The result for a sequence of nucleotides is that more than one type of polypeptide can be encoded within that sequence. (c) Nonoverlapping code In a nonoverlapping code, a single nucleotide is part of only one codon. For a sequence of RNA, this results in the production of a single type of polypeptide. (d) Initiation codon An initiation codon establishes the appropriate reading frame and specifies the first amino acid of the protein chain. Typically the initiation codon is AUG; however, both GUG and UUG can also serve as initiation codons. (e) Termination codon The termination codon signals the termination or end of translation and the end of the protein molecule. There are three types of termination codons—UAA, UAG, and UGA—which can also be referred to as stop codons or nonsense codons. These codons do not code for amino acids. (f) Sense codon A sense codon is a group of three nucleotides that code for an amino acid. There are 61 sense codons that code for the 20 amino acids commonly found in proteins. (g) Nonsense codon Nonsense codons or termination codons signal the end of translation. These codons do not code for amino acids. (h) Universal code In a universal code, each codon specifies or codes for the same amino acid in all organisms. The genetic code is nearly universal but not completely. Most of the exceptions occur in mitochondrial genes. (i) Nonuniversal codons Most codons are universal (or nearly universal) in that they specify the same amino acids in almost all organisms. However, there are exceptions where a codon has 188 Chapter Fifteen: The Genetic Code and Translation different meanings in different organisms. Most of the known exceptions are the termination codons, which in some organisms do code for amino acids. Occasionally a sense codon is substituted for another sense codon. 6. How is the reading frame of a nucleotide sequence set? The initiation codon on the mRNA sets the reading frame. 7. How are tRNAs linked to their corresponding amino acids? For each of the 20 different amino acids that are commonly found in proteins, there is a corresponding aminoacyl-tRNA synthetase that covalently links the amino acid to the tRNA molecule. 8. What role do the initiation factors play in protein synthesis? Initiation factors are proteins that are required for the initiation of translation. In bacteria, there are three initiation factors (IF1, IF2, and IF3). Each one has a different role. IF1 promotes the disassociation of the large and small ribosomal subunits. IF3 binds to the small ribosomal subunit and prevents it from associating with the large ribosomal subunit. IF2 is responsible for binding GTP and delivering the fMet-tRNAfmet to the initiator codon on the mRNA. In eukaryotes, there are more initiation factors, but many have similar roles. Some of the eukaryotic initiation factors are necessary for recognition of the 5' cap on the mRNA. Others possess a RNA helicase activity, which is necessary to resolve secondary structures. 9. How does the process of initiation differ in bacterial and eukaryotic cells? Bacterial initiation of translation requires that sequences in the 16S rRNA of the small ribosomal subunit bind to the mRNA at the ribosome binding site or Shine-Dalgarno sequence. The Shine-Dalgarno sequence is essential in placing the ribosome over the start codon (typically AUG). In eukaryotes, there is no Shine-Dalgarno sequence. The small ribosomal subunit recognizes the 5' cap of the eukaryotic mRNA with the assistance of initiation factors. Next, the ribosomal small subunit migrates along the mRNA scanning for the AUG start codon. In eukaryotes, the start codon is located with a consensus sequence called the Kozak sequence (5'–ACCAUGG–3'). Transcription in eukaryotes also requires more initiation factors. 10. Give the elongation factors used in bacterial translation and explain the role played by each factor in translation. Three elongation factors have been identified in bacteria: EF-TU, EF-TS, and EF-G. EF-TU joins with GTP followed by a tRNA charged with an amino acid. The charged tRNA is delivered to the ribosome at the “A” site. During the process of delivery, the GTP joined to EF-TU is cleaved to form a EF-TU-GDP complex. EF-TS is necessary to regenerate EF-TU-GTP. The elongation factor EF-G binds GTP and is necessary for the translocation or movement of the ribosome along the mRNA during translation. 11. What events bring about the termination of translation? The process of termination begins when a ribosome encounters a termination codon. Since the termination codon would be located at the “A” site, no corresponding tRNA Chapter Fifteen: The Genetic Code and Translation 189 will enter the ribosome. This allows for the release factors (RF1, RF2, and RF3) to bind the ribosome. RF1 recognizes and interacts with the stop codons UAA and UAG, while RF2 can interact with UAA and UGA. A RF3-GTP complex binds to the ribosome. Termination of protein synthesis is complete when the polypeptide chain is cleaved from the tRNA located at the “P” site. During this process, the GTP is hydrolyzed to GDP. 12. Give several examples of RNA-RNA interactions that take place in protein synthesis. Several RNA-RNA interactions that take place during protein synthesis are important. The tRNA molecules form base pairs with codons on the mRNA. The 3' end of the 16S rRNA within the small ribosomal subunit forms base pairs with Shine-Dalgarno sequence at the 5' end of the mRNA. Ribosomal RNAs on both the large and small subunit interact with tRNAs at both the “A” and the “P” sites. The association of the large and small subunits of the ribosome is potentially the result of interactions between the 16S rRNA of the small subunit and the 23S rRNA of the large subunit. 13. What are some types of posttranslational modification of proteins? Several different modifications can occur to a protein following translation. Frequently the amino terminal methionine may be removed. Sometimes in bacteria only the formyl group is cleaved from the N-formyl methionine, leaving a methionine at the amino terminal. More extensive modification occurs in some proteins that are originally synthesized as precursor proteins. These precursor proteins are cleaved and trimmed by protease enzymes to produce a functional protein. Glycoproteins are produced by the attachment of carbohydrates to newly synthesized proteins. Molecular chaperones are needed by many proteins to ensure that the proteins are folded correctly. Secreted proteins that are targeted for the membrane or other cellular locations frequently have 15 to 30 amino acids, called the signal sequence, removed from the amino terminal. Finally, acetylation of amino acids in the amino terminal of some eukaryotic proteins also occurs. 14. Explain how some antibiotics work by affecting the process of protein synthesis. A number of antibiotics bind the ribosome and inhibit protein synthesis at different steps in translation. Some antibiotics such as streptomycin bind to the small subunit and inhibit translation initiation. Other antibiotics such as chloramphenicol bind to the large subunit and block elongation of the peptide by preventing peptide bond formation. Antibiotics such as tetracycline and neomycin bind the ribosome near the “A”’ site yet have different effects. Tetracyclines block entry of charged tRNAs to the “A” site, while neomycin induces translational errors. Finally, some antibiotics such as erythomycin block the translocation of the ribosome along the mRNA. 15. Compare and contrast the process of protein synthesis in bacterial and eukaryotic cells, giving similarities and differences in the process of translation in these two types of cells. Bacterial and eukaryotic cells share several similarities as well as have several differences in protein synthesis. Initially bacteria and eukaryotes share the universal genetic code. However, the initiation codon, AUG, in eukaryotic cells codes for 190 Chapter Fifteen: The Genetic Code and Translation methionine, whereas in bacteria the AUG codon codes for N-formyl methionine. In eukaryotes, transcription takes place within the nucleus, whereas most translation takes place in the cytoplasm (although some translation does take place within the nucleus). So, transcription and translation in eukaryotes are kept temporally and spatially separate. However, in bacterial cells transcription and translation occur nearly simultaneously. Stability of mRNA in eukaryotic cells and bacterial cells is also different. Bacterial mRNA is typically short lived, lasting only a few minutes. Eukaryotic mRNA may last hours or even days. Charging of the tRNAs with amino acids is essentially the same in both bacteria and eukaryotes. The ribosomes of bacteria and eukaryotes are different as well. Both bacteria and eukaryotes have large and small ribosomal subunits, but they differ in size and composition. The bacterial large ribosomal consists of two ribosomal RNAs, while the eukaryotic large ribosomal subunit consists of three. During translation initiation, the bacterial small ribosomal subunit recognizes the Shine-Dalgarno consensus sequence in the 5' UTR of the mRNA and to regions of the 16S rRNA. In most eukaryotic mRNAs, the small subunit binds the 5' cap of the mRNA and scans downstream until it encounters the first AUG codon. Finally, elongation and termination in bacterial and eukaryotic cells are functionally similar, although different elongation and termination factors are used. APPLICATION QUESTIONS AND PROBLEMS 16. Sydney Brenner isolated Salmonella typhimurium mutants that were implicated in the biosynthesis of tryptophan and would not grow on minimal medium. When these mutants were tested on minimal medium to which one of four compounds (indole glycerol phosphate, indole, anthranilic acid, and tryptophan) had been added, the growth responses shown in the table on the facing page were obtained. Give the order of indole glycerol phosphate, indole, anthranilic acid, and tryptophan in a biochemical pathway leading to the synthesis of tryptophan. Indicate which step in the pathway is affected by each of the mutations. Chapter Fifteen: The Genetic Code and Translation 191 Mutant Minimal Anthranilic medium acid Indole glycerol phosphate Indole Tryptophan trp-1 – – – – + trp-2 – – + + + trp-3 – – – + + trp-4 – – + + + trp-6 – – – – + trp-7 – – – – + trp-8 - + + + + trp-9 – – – – + trp-10 – – – – + trp-11 – – – – + Based on the mutant strain’s ability to grow on the above substrates, we can group the mutations into 4 groups that we will call group 1, group 2, group 3, and group 4. Group 1 mutants can only grow on the minimal medium supplemented with trpytophan. Group 1: trp 1, trp 10, trp 11, trp 9, trp 6, and trp 7. Group 2 mutants can grow on the minimal medium supplemented with either trpytophan or indole. Group 2: trp 3. Group 3 mutants can grow on the minimal medium supplemented with tryptophan, indole, or indole glycerol phosphate. Group 3: trp 2 and trp 4. Group 4 mutants can grow on minimal medium supplemented with the addition of tryptophan, indole, indole glycerol phosphate, or anthranilic acid. Group 4: trp 8. By examining the compounds needed for growth by the different groups of mutants, we can identify the step in the pathway that is blocked by each mutation. For each group, the pathway step blocked will correspond to the step proceeding the last compound on which a mutant strain can grow. Any compound added to the minimal media that proceeds the block will not allow for growth of the mutant strain. Group 1 mutants can only grow when tryptophan is added to the growth medium. So group 1 mutants are blocked at the last step in the biosynthesis process before tryptophan is synthesized. Since group 2 mutants can grow with either tryptophan or indole added to the growth medium, this suggests that in the pathway indole is the immediate precursor of tryptophan and that group 2 mutants are blocked in the step proceeding the synthesis of indole. Using the same type of analysis, we can create the pathway on the following page for synthesis of tryptophan. 192 Chapter Fifteen: The Genetic Code and Translation Group 4 Group 3 Group 2 Group 1 Precursor anthranilic Indole glyerol Indole Tryptophan acid phosphate 17. The addition of a series of compounds yielded the following biochemical pathway: precursor compound I compound II compound III enzyme A enzyme B enzyme C Mutation a inactivates enzyme A, mutation b inactivates enzyme B, and mutation c inactivates enzyme C. Mutants, each having one of these defects, were tested on minimal medium to which compound I, II, and III was added. Fill in the results expected of these tests by placing a plus sign (+) for growth or a minus sign (–) for no growth in the following table: Minimal medium to which is added Strain with mutation Compound I Compound II a + + b – + c – – Compound III + + + To determine whether growth will occur on the minimal medium with the added compound, the step in the pathway where the mutation occurs must be considered. Since mutation a affects enzyme A, then any strain with mutation a can only grow on minimal media with the addition of compound I, compound II or compound III. Strains containing mutation b can only grow with the addition of either compound II or compound III because enzyme B, which converts compound I to compound II, has been affected. Strains with mutation c can only grow with the addition of compound III since the enzyme needed to synthesize compound III from compound II has been mutated. 18. Assume that the number of different types of bases in RNA is four. What would be the minimum codon size (number of nucleotides) required if the number of different types of amino acids in proteins were: (The number of codons possible must be equal to or greater than the number of different types of amino acids since the codons encode for the different amino acids. To calculate how many possible codons that are possible for a given codon size with four different types of bases in the RNA, the following formula can be used: 4n, where n is the number of nucleotides within the codon.) (a) 2 1, because 41 = 4 codons, which is more than enough to specify 2 different amino acids. (b) 8 2. (c) 17 3. Chapter Fifteen: The Genetic Code and Translation 193 (d) 45 3. (e) 75 4. 19. How many codons would be possible in a triplet code if only three bases (A, C, and U) were used? To calculate the number of possible codons of a triplet code if only three bases are used, the following equation can be used: 3n, where n is the number of nucleotides within the codon. So, the number of possible codons is equal to 33, or 27 possible codons. 20. Using the genetic code given in Figure 15.14, give the amino acids specified by the bacterial mRNA sequences and indicate the amino and carboxyl ends of the polypeptide produced. Each of the mRNA sequences begins with the three nucleotides AUG. This indicates the start point for translation and allows for a reading frame to be set. In bacteria, the AUG initiation codon codes for N-formyl-methionine. Also for each of these mRNA sequences, a stop codon is present either at the end of the sequence or within the interior of the sequence. The amino terminal refers to the end of the protein with a free amino group and will be the first peptide in the chain. The carboxyl terminal refers to the end of the protein with a free carboxyl group and is the last amino acid in the chain. For the following peptide chains reading from left to right, the first amino acid is located at the amino end, while the last amino acid is located at the carboxyl end. (a) 5'–AUGUUUAAAUUUAAAUUUUGA–3' Amino fMet–Phe–Lys–Phe–Lys–Phe Carboxyl (b) 5'–AUGUAUAUAUAUAUAUGA--3' Amino fMet–Tyr–Ile--Tyr--Ile Carboxyl (c) 5'–AUGGAUGAAAGAUUUCUCGCUUGA–3' Amino fMet–Asp–Glu–Arg–Phe–Leu–Ala Carboxyl (d) 5'–AUGGGUUAGGGGACAUCAUUUUGA–3' Amino fMet–Gly Carboxyl (The stop codon UAG occurs after the codon for glycine.) 21. A nontemplate strand on DNA has the following base sequence. What amino acid sequence would be encoded by this sequence? 5'–ATGATACTAAGGCCC–3' To determine the amino acid sequence, we need to know the mRNA sequence and the codons present. The nontemplate strand of the DNA has the same sequence as the mRNA except that thymine containing nucleotides are substituted for the uracil containing nucleotides. So the mRNA sequence would be as follows: 5'–AUGAUACUAAGGCCC–3'. Assuming that the AUG indicates a start codon, then the amino acid sequence would be starting from the amino end of the peptide and ending with the carboxyl end: fMet–Met–Leu–Arg–Pro 194 Chapter Fifteen: The Genetic Code and Translation 22. The following amino acid sequence is found in a tripeptide: Met–Trp–His. Give all possible nucleotide sequences on the mRNA, on the template strand of DNA, and on the nontemplate strand of DNA that could encode this tripeptide. The potential mRNA nucleotide sequences encoding for the tripeptide Met–Trp–His can be determined by using the codon table found in Figure 15.14. From the table, we can see that the amino acid His has two potential codons, while the amino acids Met and Trp each have only one potential codon. So, there are two different mRNA nucleotide sequences that could encode for the tripeptide. Once the potential mRNA nucleotide sequences have been determined, the template and nontemplate DNA strands can be derived from these potential mRNA sequences. (1) 5'–AUGUGGCAU–3' DNA template: DNA nontemplate: (2) 5'–AUGUGGCAC–3' DNA template: DNA nontemplate: 3'–TACACCGTA–5' 5'–ATGUGGCAT–3' 3'–TACACCGTG–5' 5'–ATGTGGCAC–3' 23. How many different mRNA sequences can code for a polypeptide chain with the amino acid sequence Met–Leu–Arg? (Be sure to include the stop codon.) From Figure 15.14, we can determine that leucine and arginine each have six different potential codons. There are also three potential stop codons. As for methionine, only one codon, AUG, is typically found as the initiation codon. (However, both UUG and GUG have been shown to serve as start codons on occasion. For this problem, we will ignore these rare cases.) So, the number of potential sequences is the product of the number of different potential codons for this tripeptide, which gives us a total of (1 × 6 × 6 × 3) = 108 different mRNA sequences that can code for the tripeptide Met–Leu– Arg. 24. A series of tRNAs have the following anticodons. Consider the wobble rules given in Table 15.2, and give all possible codons with which each tRNA can pair. From the wobble rules outlined in Table 15.2, we can see that when “A” occurs at the 5' of the anticodon it can only pair with “U” in the 3' end of the codon. When “C” is present at the 5' of the anticodon, it can only pair with “G” at the 3' of the codon. However, both “U” and “G” when present at the 5' end of the anticodon can pair with two different nucleotides at the 3' end of the codon (U with A or G; and G with U or C). The rare base iosine (I) is also found at the 5' of the anticodon of tRNA on occasion. Iosine can pair with “A”, “U,” or “C” at the 3' end of the codon. (a) 5'–GGC–3' Codons: 3'–CCG–5' or 3'–UCG–5'. (b) 5'–AAG–3' Codon: 3'–UUC–5'. (c) 5'–IAA–3' Codons: 3'–AUU–5' or 3'–UUU–5' or 3'–CUU–5'. (d) 5'–UGG–3' Codons: 3'–ACC–5' or 3'–GCC–5'. Chapter Fifteen: The Genetic Code and Translation 195 (e) 5'–CAG–3' Codon: 3'–GUC–5'. 25. An anticodon on a tRNA has the sequence 5'–GCA–3'. (a) What amino acid is carried by this tRNA? The anticodon 5'–GCA–3' would pair with the codon 5'–CGU–3'. Based on the codon table in Figure 15.14, the amino acid encoded by this codon is cysteine. So, this tRNA is most likely carrying cysteine. (b) What would be effect if the G in the anticodon were mutated to a U? The anticodon would now be 3'–ACU–5' and could pair to the codon 5'–UGA–3', a stop codon. The result would be that amino acid cysteine would be placed where the stop codon 5'–UGA–3' was located in the mRNA. Essentially, the stop codon would be suppressed and translation could continue. 26. Which of the following amino acid changes could result from a mutation that changed a single base? For each change that could result from the alteration of a single base, determine which position of the codon (first, second or third nucleotide) in the mRNA must be altered for the change to occur. (a) Leu Gln Of the six codons that encode for Leu, only two could be mutated by the alteration of a single base to produce the codons for Gln: CUA (Leu)—Change the second position to A to produce CAA (Gln). CUG (Leu)—Change the second position to A to produce CAG (Gln). (b) Phe Ser Both Phe codons (UUU and UUC) could be mutated at the second position to produce Ser codons: UUU (Phe)—Change the second position to C to produce UCU (Ser). UUC (Phe)—Change the second postion to C to produce UCC (Ser). (c) Phe Ile Both Phe codons (UUU and UUC) could be mutated at the first position to produce Ile codons: UUU (Phe)—Change the first position to A to produce AUU (Ile). UUC (Phe)—Change the first position to A to produce AUC (Ile). (d) Pro Ala All four codons for Pro can be mutated at the first position to produce Ala codons: CCU (Pro)—Change the first position to G to produce GCU (Ala). CCC (Pro)—Change the first position to G to produce GCC (Ala). CCA (Pro)—Change the first position to G to produce GCA (Ala). CCG (Pro)—Change the first position to G to produce GCG (Ala). (e) Asn Lys Both codons for Asn can be mutated at a single position to produce Lys codons: AAU (Asn)—Change the third position to A to produce AAA (Lys). AAU (Asn)—Change the third position to G to produce AAG (Lys). AAC (Asn)—Change the third postion to A to produce AAA (Lys). AAC (Asn)—Change the third position to G to produce AAG (Lys). 196 Chapter Fifteen: The Genetic Code and Translation (f) Ile Asn Only two of the three Ile codons can be mutated at a single position to produce Asn codons: AUU (Ile)—Change the second position to A to produce AAU (Asn). AUC (Ile)—Change the second position to A to produce AAC (Asn). 27. A synthetic mRNA added to a cell-free protein-synthesizing system produces a peptide with the following amino acid sequence: Met–Pro–Ile–Ser–Ala. What would be the effect on translation if the following components were omitted from the cell-free protein-synthesizing system? What, if any, type of protein would be produced? Explain your reasoning. (a) Initiation factor 1 The lack of IF1 would decrease the amount of protein synthesized. IF1 promotes the disassociation of the large and small ribosomal subunits. Translation initiation would occur, but at a slower rate since more of the small ribosomal subunits would be bound to the large ribosomal subunits. (b) Initiation factor 2 No translation would occur. IF2 is necessary for translation initiation. The lack of IF2 would prevent fMet-tRNAfmet from being delivered to the small ribosomal subunit, thus blocking translation. (c) Elongation factor Tu Although translation initiation or delivery of the Met to the ribosome-mRNA complex would occur, no further amino acids would be delivered to the ribosome. EF-TU is necessary for elongation where it binds GTP and the charged tRNA. This three-part complex enters the “A” site of the ribosome. If EF-TU is not present, then the charged tRNA will not enter the “A” site, thus stopping translation. (d) Elongation factor G EF-G is necessary for the translocation of the ribosome along the mRNA in a 5' to 3' direction. Once the formation of the peptide bond occurs between the Met and Pro, the lack of EF-G would prevent the movement of the ribosome along the mRNA, so no new codons would be read. However, the dipeptide Met-Pro would be formed since its formation would not require EF-G. (f) Release factors R1, R2, and R3 The release factors recognize the stop codons and promote cleavage of the peptide from the tRNA at the “P” site. The absence of the release factors prevents termination of translation at the stop codon, resulting in a larger peptide. (g) ATP ATP is required for the charging of the tRNAs with amino acids by the aminoacyltRNA synthetases. Without ATP, the charging would not take place, and no amino acids will be available for protein synthesis. So no protein synthesis will occur. (h) GTP GTP is required for initiation, elongation, and termination of translation. If GTP is absent, no protein synthesis will occur. Chapter Fifteen: The Genetic Code and Translation 197 CHALLENGE QUESTIONS 28. In what ways are spliceosomes and ribosomes similar? In what ways are they different? Can you suggest some possible reasons for their similarities? Spliceosomes and ribosomes are both large complexes that are composed of several different RNA and protein molecules. In essence, both the spliceosome and ribosome are RNA-based enzymes or ribozymes. The RNA molecules in both structures are necessary for catalysis. The 23S RNA molecule in the ribosome catalyzes the formation of peptide bonds between amino acids. Other rRNAs are also important for protein synthesis. In spliceosomes, the snRNA molecules catalyze the cutting and splicing of pre-mRNA molecules to produce mature mRNA. The catalytic RNAs of the ribosome and the spliceosome may have originated during the RNA world when RNA molecules served to store information and to catalyze reactions that sustained life. 29. Several experiments were conducted to obtain information about how the eukaryotic ribosome recognizes the AUG start codon. In one experiment, the gene that codes for methionine initiator tRNA (tRNAiMet) was located and changed. The nucleotides that specify the anticodon on tRNAiMet were mutated so that the anticodon in the tRNA was 5'–CCA–3' instead of 5'–CAU–3'. When this mutated gene was placed into a eukaryotic cell, protein synthesis took place, but the proteins produced were abnormal. Some of the proteins produced contained extra amino acids, and others contained fewer amino acids. (a) What do these results indicate about how the ribosome recognizes the starting point for translation in eukaryotic cells? Explain your reasoning. By mutating the anticodon to 5'–CCA–3' from 5'–CAU–3' on tRNAiMet, the initiator tRNA will now recognize the codon 5'–UGG–3', which normally would code only for Trp. If translation initiation by the ribosome in eukaroytes occurs by binding the 5' cap of the mRNA followed by scanning, then the first 5'–UGG–3' codon recognized by the mutated tRNAiMet will be the start site for translation. If the first 5'–UGG–3' codon occurs prior to the normal 5'–AUG–3' codon, then a protein containing extra amino acids could be produced. If the first 5'–UGG–3' codon occurs after the normal 5'–AUG–3', then a shorter protein will be produced. Finally, truncated proteins could also be produced by the first 5'–UGG–3' being out of frame of the normal coding sequence. If this happens, then most likely a stop codon will be encountered before the end of the normal coding sequence and will terminate translation. The data suggest that translation initiation takes place by scanning of the ribosome for the appropriate start sequence. (b) If the same experiment had been conducted on bacterial cells, what results would you expect? Very little or no protein synthesis would be expected. Translation initiation in bacteria requires the 16S RNA of the small ribosomal subunit to interact with the Shine-Dalgarno sequence. This interaction serves to line up the ribosome over the start codon. If the anticodon has been changed such that the start codon cannot be recognized, then protein synthesis is not likely to take place.