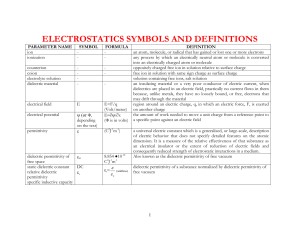
Dielectric Permittivity and Thermophysical Properties
advertisement

Dielectric Permittivity and Thermophysical Properties of Carbon Dioxide in Critical Point Region Onishchenko V.P., Zhelezny V.P., Dzhenkov M.Yu. Odessa State Academy of Refrigeration Construction of theoretically proved equations of a condition of gases and liquids is of interest for the solution of a number of practical technological problems, in particular, designing the gas extraction processes in overcritical region of parameters. The thermodynamic perturbation theory allows reaching the prediction of thermodynamic properties of polar and non-polar gases and liquids within the error, close to an error of experimental data. Thus was used the Lennard - Jones potential with the parameters dependent on temperature [1]. The collective phenomena, which start to manifest in gases of moderate density and grow in liquid, result in screening of intermolecular interaction potential. These phenomena are fully taken into account within the thermodynamic perturbation theory for the description of thermal and caloric properties in single-phase region. However they are not fully taken into account for the description of properties within near-critical regions of a liquid - vapour equilibrium. Similarly, these collective phenomena and intermolecular interaction potential screening detetermine dependences of dielectric permittivity on temperature and density. This work presents the results of simulation of dependence both of thermodynamic properties and of dielectric permittivity of carbon dioxide within near-crytical area of state parameters. Screening parameters enter relations of thermodynamic perturbation theory in the formulation [1] and on semi-empirical basis they are connected to dielectric permittivity. Results of detailed comparison to experimental data [2] for carbon dioxide are presented. For example, the error of the description of pressure on near-critical isotherms does not exceed 0,1 МPа. 1. Onishchenko V.P., Kutirkin O.F., Zhelezny V.P., Vladimirov B.P. Thermodynamic properties of polar fluids: ozone - safe refrigerants in gaseous and liquid states // High Temperatures - High Pressures, GB. 1997. - Vol. 29. - p. 313 - 318. 2. Span R., Wagner W. A New Equation of State for Carbon Dioxide // J. Phys. Chem. Ref. Data - 1996. Vol. 25, No. 6. - p. - 1520.