Thermodynamics Revision
advertisement
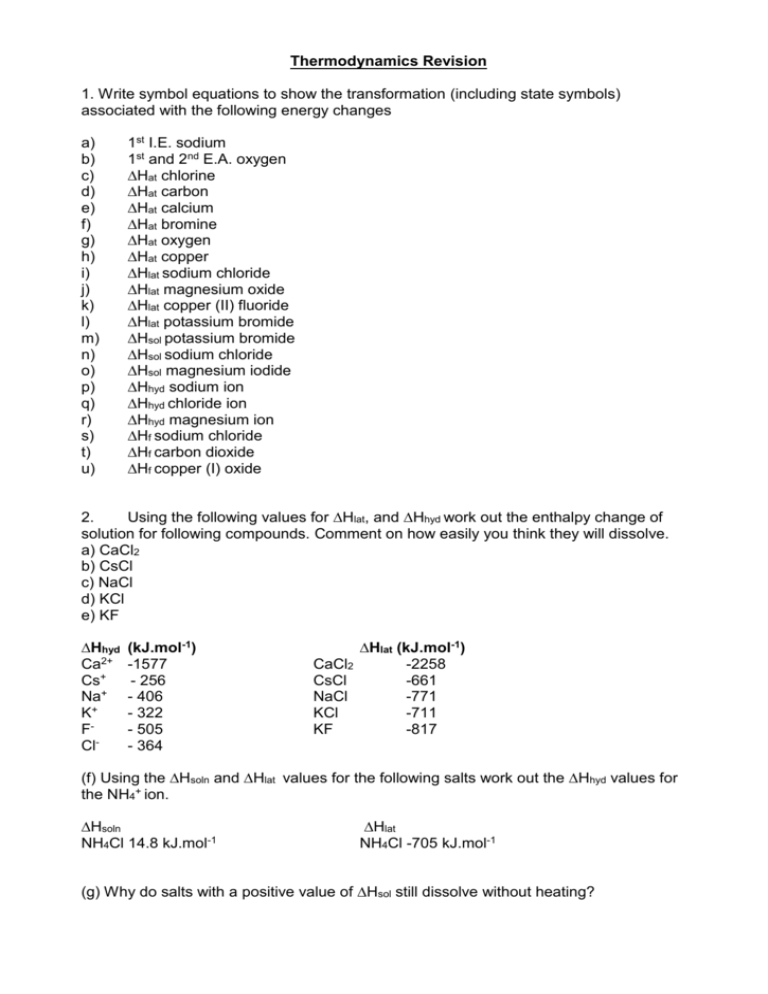
Thermodynamics Revision 1. Write symbol equations to show the transformation (including state symbols) associated with the following energy changes a) b) c) d) e) f) g) h) i) j) k) l) m) n) o) p) q) r) s) t) u) 1st I.E. sodium 1st and 2nd E.A. oxygen ∆Hat chlorine ∆Hat carbon ∆Hat calcium ∆Hat bromine ∆Hat oxygen ∆Hat copper ∆Hlat sodium chloride ∆Hlat magnesium oxide ∆Hlat copper (II) fluoride ∆Hlat potassium bromide ∆Hsol potassium bromide ∆Hsol sodium chloride ∆Hsol magnesium iodide ∆Hhyd sodium ion ∆Hhyd chloride ion ∆Hhyd magnesium ion ∆Hf sodium chloride ∆Hf carbon dioxide ∆Hf copper (I) oxide 2. Using the following values for ∆Hlat, and ∆Hhyd work out the enthalpy change of solution for following compounds. Comment on how easily you think they will dissolve. a) CaCl2 b) CsCl c) NaCl d) KCl e) KF ∆Hhyd Ca2+ Cs+ Na+ K+ FCl- (kJ.mol-1) -1577 - 256 - 406 - 322 - 505 - 364 ∆Hlat (kJ.mol-1) CaCl2 -2258 CsCl -661 NaCl -771 KCl -711 KF -817 (f) Using the ∆Hsoln and ∆Hlat values for the following salts work out the ∆Hhyd values for the NH4+ ion. ∆Hsoln NH4Cl 14.8 kJ.mol-1 ∆Hlat NH4Cl -705 kJ.mol-1 (g) Why do salts with a positive value of ∆Hsol still dissolve without heating? 3. Construct a Born-Haber cycle and using the data supplied 1. Calculate ∆Hf for LiCl(s) 2. Calculate ∆Hlat for MgO(s) 3. Calculate 1st E.A. for F(g) → F-(g) 4. Calculate 1st I.E. for K(g) → K+(g) 5. Calculate ∆Hat for silver. ∆Hf values (kJ.mol-1) MgO -602 CaF2 -1220 KBr -394 AgCl -127 ∆Hlat values (kJ.mol-1) CaF2 -2630 KBr -679 LiCl -848 AgCl -905 ∆Hat values (kJ.mol-1) Li 159 Mg 150 Ca 178 K 89 Bond enthalpy values (kJ.mol-1) F-F 158 Cl-Cl 244 Br-Br 194 (note ∆vap = 30) O=O 498 Ionisation energy (I.E.) values (kJ.mol-1) Li 520 Mg 738 1455 Ca 590 1145 Ag 731 Electron affinities (E.A.) values (kJ.mol-1) Cl -349 Br -325 O -141 798 4. Using the following values for the enthalpy of formation (kJ.mol-1) of two related compounds comment on which is the most stable (least reactive). Try to write an equation showing the conversion of one into the other and calculate the energy change for that reaction using Hess’ Law. Is it exothermic or endothermic? Could you have predicted that beforehand? H2O2 H2O -188 -286 LiH LiOH -90 -485 AlCl3 Al(OH)3 -704 -1676 5. Do you expect the entropy (∆Ssys) to increase or decrease in these reactions? Why? a) b) c) d) e) f) 3H2(g) + N2(g) 2H2(g) + O2(g) 2H2O2(l) → 2Na(s) + Cl2(g) CaCO3(s) → 3Fe(s) + 2O2(g) → 2NH3(g) → 2H2O(l) 2H2O(l) + O2(g) → 2NaCl(s) CaO(s) + CO2(g) → Fe3O4(s) 6. Use the following values for S (J.K-1.mol-1) to calculate actual values of ∆Ssys for the reactions a) to f) above. H2 130.6 N2 191.6 O2 205.0 Cl2 165.0 H2O(l) 69.9 H2O2(l) 109.6 Na 51.2 NaCl(s) 72.1 CaO(s) 39.7 CaCO3(s) 92.9 CO2(g) 213.6 Fe 27.3 Fe3O4(s) 146.4 NH3(g) 192.3 7. Using ∆Hf values in the data book calculate ∆Hr values for the above reactions. In some cases you will need to construct Hess cycles. According to the second law of thermodynamics entropy always increases, but in reactions a), b) d) and f) above it decreases. Explain. 8. Calculate ∆G values at 273K for each reaction and comment on its feasibility at that temperature. 9. What will happen for those reactions with a drop in Ssys, as the temperature increases? 10. If any reactions are not feasible at 273K, what temperature do they become feasible? 11. Find out at what temperature the following reactions become feasible. a) ZnO(s) + C(s) → b) Fe3O4 (s) + 4H2(g) → c) 2Mg(s) + O2(g) d) 2Ag2O(s) → e) BaCO3(s) → → ZnO(s) + CO(g) ∆H= +237 kJ.mol-1, ∆S= + 189.9 J.K-1.mol-1 3Fe(s) + 4H2O(9) ∆H= -144 kJ.mol-1, ∆S= + 226.9 J.K-1.mol-1 2MgO(s) ∆H= -1203 kJ.mol-1, ∆S= - 216.6 J.K-1.mol-1 4Ag(s) + O2(g) ∆H= +62 kJ.mol-1, ∆S= + 132.8 J.K-1.mol-1 BaO(s) + CO2(g) ∆H= +268 kJ.mol-1, ∆S= + 171.9 J.K-1.mol-1 12. Some workers transform a field full of stones into dry stone walls surrounding the field. How has the entropy changed with respect to the arrangement of stones? How has the overall entropy of this process increased according to the second law of thermodynamics? Mark Scheme c) d) e) f) g) h) i) j) k) l) m) n) o) p) q) r) s) t) u) Na(g) → Na(g) O2(g) + e → O-(g) + e→ ½ Cl2(g) → C(s) → C(g) Ca(s) → ½ Br(l) → ½ O2(g) → Cu(s) → Cu(g) Na+(g) + Cl-(g) Mg2+(g) + O2-(g) Cu2+(g) + 2F-(g) K+(g) + Br-(g) KBr(s) → NaCl(s) → MgI2(s) → + Na (g) → Cl-(g) → Mg2+(g) → Na(s) + ½ Cl2(g) C(s) + O2(g) → 2Cu(s) + ½ O2(g) 2. a) a) b) + eO-(g) O2-(g) Cl(g) Ca(g) Br(g) O(g) → NaCl(s) → MgO(s) → CuF2(s) → KBr(s) K+(aq) + Br-(aq) Na+(aq) + Cl-(aq) Mg2+(aq) + 2I-(aq) Na+(aq) Cl-(aq) Mg2+(aq) → NaCl(s) CO2(g) → Cu2O(s) ∆Hsol = ∑∆Hhyd - ∆Hlat = (-1577) + 2(-364) – (-2258) = - 47 kJ.mol-1 b) ∆Hsol = (-256) + (-364) – (-661) = + 41 kJ.mol-1 c) ∆Hsol = (-406) + (-364) – (-771) = +1 kJ.mol-1 d) ∆Hsol = (-322) + (-364) – (-711) = + 25 kJ.mol-1 e) ∆Hsol = (-322) + (-505) – (-817) = -10 kJ.mol-1 Initial thoughts are that those that dissolve with an exotherm a) and e) or very small endotherm c) would be the most soluble. However CsCl despite its significant endotherm (+41 kJ.mol-1) is readily soluble. f) ∆Hsol = ∑∆Hhyd - ∆Hlat 14.8 = (-364) + ∆Hhyd(ammonium) – (-705) ∆Hhyd(ammonium) = - 326.2 kJ.mol-1 g) This is because formation of solutions from solids always a significant increase in entropy. 3. a) ∆Hf b) -602 = 150 + 249 + 738 + 1455 – 141 + 798 +∆Hlat ∆Hlat = - 3851 kJ.mol-1 c) -1220 = 178 + 158 + 178 + 590 + 1145 + 2(E.A.) - 2630 E.A. = - 330.5 kJ.mol-1 d) - 394 = 89 + 112 + I.E. – 325 – 679 I.E. = + 409 kJ.mol-1 e) -127 = ∆Hat + 122 + 731 – 349 – 905 ∆Hat = + 274 kJ.mol-1 a) H2O2 → 4. = 159 + 122 + 520 – 349 – 848 = - 396 kJ.mol-1 H2O + ½ O2 -286 ↑ -188 Elements in their standard states b) c) ∆Hr = -286-(188) = -98 kJ.mol-1 LiH + H2O ∆Hr = -485-(-90-286) = -109 kJ.mol-1 AlCl3 + 3 H2O ∆Hr → LiOH + H2 → Al(OH)3 + 3HCl = -3(286)-704-(-1676-3(93)) = -393 kJ.mol-1 (∆Hf(HCl)= -93 kJ.mol-1) Compounds with a highly negative value of ∆Hf are more stable or less reactive. This is illustrated above by the fact that a related more reactive compound can be converted into a more stable form in an exothermic reaction. 5. a) b) c) d) e) f) decrease (4 to 2 molecules) decrease (3 gas to one liquid) increase (2 to 3 molecules and l to g) decrease ( g to s ) increase ( s to g) decrease (g to s) 6. 7. = ∆S (J.K-1.mol-1) - 198.8 a) Sproducts 384.6 - Sreactants 583.4 b) 139.8 - 466.2 - 326.4 c) 344.8 - 219.2 + 125.6 d) 144.2 - 267.4 -123.2 e) 253.3 - 92.9 + 160.4 f) 146.4 - 491.9 - 345.5 a) -92.3 kJ.mol-1 b) -571.6 kJ.mol-1 c) -196.0 kJ.mol-1 d) -822.4 kJ.mol-1 e) +178.3 kJ.mol-1 f) – 1118.4 kJ.mol-1 a), b), d) and f) are exothermic so heat energy is transferred to the surroundings meaning the entropy of the surroundings will be increased. 8. a) -38.03 kJ.mol-1 b) -482.5 kJ.mol-1 c) –230.3 kJ.mol-1 d) -788.8 kJ.mol-1 e) +134.2 kJ.mol-1 f) – 1024.1.0 kJ.mol-1 feasible at 273K feasible at 273K feasible at 273K feasible at 273K not feasible at 273K feasible at 273K 9. If the products are becoming more ordered (decrease in entropy) the T∆S term becomes more positive as temperature increases. At sufficiently high temperatures the reaction will become unfeasible as it will cancel out the exotherm. 10. Becomes feasible at 1110K 11. a) T = 237/0.1899 = 1248K b) exothermic with increase in entropy – always feasible c) exothermic with drop in entropy feasible up to 5554K d) T= 62/0.1328 = 467K e) T = 268/ 0.01719 = 1559K 12. The stones have fewer ways or being arranged (more ordered) and therefore their entropy has been reduced. The workers have had to respire to produce this ordering so glucose molecules have been converted into CO2 and water molecules, and heat has been generated so the overall entropy of the surroundings has increased.







