Technetium (99mTc) DTPA Injection [Technetium (99mTc) Pentetate
advertisement

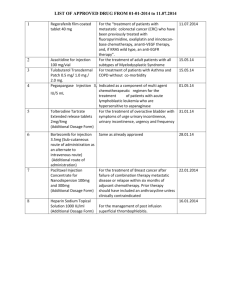
Technetium (99mTc) DTPA Injection Technetium (99mTc) Pentetate Injection Category. Diagnostic. Description. A clear, colourless solution. Technetium (99mTc) Pentetate Injection contains not less than 90.0 per cent and not more than 110.0 per cent of the stated technetium-99m radioactivity at the date and time stated on the label. Not less than 95.0 per cent of the radioactivity corresponds to technetium-99m complexed with sodium DTPA Radiopharmaceutical preparation. Technetium (99mTc) Pentetate Injection is prepared aseptically following manufacturer’s instructions provided with the kit. The kit contains sodium diethylenetriaminepenta-acetate, stannous salt and may contain reducing, chelating, stabilizing, filling and antioxidizing agents, as well as antimicrobial preservatives and buffers. Sodium pertechnetate 99mTc (fission or non-fission), complying with specification given for it, is used with the kit to make the ( 99m Tc) Pentetate Injection. Identification A. Determine by gamma-ray spectrometry. The most prominent gamma photon of technetium-99m has an energy of 0.140 MeV. B. In the test for Radiochemical purity, the chromatogram obtained contributes to the identification of the distribution of radioactivity in the preparation. C. Place in a clean, dry 10 ml glass tube a volume of the injection under examination containing 2 mg of pentetate. Dilute, if necessary, to 1 ml with water. Place in a second tube 1 ml of water (blank). To each tube add 0.1 ml of a 0.1 per cent w/v solution of nickel sulphate, 0.5 ml of a 50 per cent v/v solution of glacial acetic acid and 0.75 ml of a 5.0 per cent w/v solution of sodium hydroxide. Mix and verify that the pH is not above 5. To each tube add 0.1 ml of a 1.0 per cent w/v solution of dimethylglyoxime in ethanol (95 per cent). Mix and allow to stand for 2 minutes. Adjust the pH in each tube to not less than 12 by adding a 10.0 per cent w/v solution of sodium hydroxide. Mix and check that the pH is not below 12. Allow to stand for 2 minutes. Heat the tubes gently on a water-bath for 2 minutes. The solution in the tube containing the injection under examination remains clear and colourless throughout. The solution in the blank tube becomes red on addition of dimethylglyoxime solution and a red precipitate is formed when the tube is heated on a water-bath. Tests pH (2.4.24). 5.0 to 8.0. Radioactivity assay. Radioactivity of an aliquot of Test Solution is assayed using precalibrated radioactivity dose calibrator as described in measurement of radioactivity in general chapter on Radiopharmaceutical preparation. It is expressed as MBq(mCi) in volume V ml at a particular time and date. Radionuclidic purity. Not less than 99.9 per cent due to Technetium-99m. Complies with the radionuclidic purity tests as described under monograph on Sodium Pertechnetate (99mTc) Injection (Fission) or Sodium Pertechnetate (99mTc) Injection (Non Fission) used for preparation of Technetium (99mTc) Pentetate Injection. Radiochemical purity. Method A Determine the RCP of the injection by paper chromatography Support: Whatman chromatography paper No 1 Solvent: 85 % methanol Spot about two to five microlitres of 99mTc- DTPA Injection with a capillary or a micropipette at the point of spotting marked at 3 cm from one end of the strip (3 X 23 cm) in duplicate. Develop the chromatogram by ascending chromatography till the solvent front reaches 15 cm from the point of spotting. This takes about 90 min. Allow the strip to dry. Scan the strip using a radiochromatogram scanner with collimator suitable adjusted to measure 99mTc or cut into one cm sections and count in a well type NaI(Tl) scintillation counter. The Rf values of 99mTc-DTPA and 99m Tc-pertechnetate are 0.0-0.5 and 0.55-0.7 respectively. Calculate the % of activity present as 99mTc-DTPA as follows: (%) RCP = Activity in the 99mTc-DTPA zone x 100 / Total Activity The activity in the 99mTc-DTPA zone shall not be less than 90% of the total activity. Method B Determine by thin-layer chromatography (2.4.17), silica gel G coated plates. Mobile phase (a): A 0.9 per cent w/v solution of sodium chloride in water. Mobile phase (b): Methyl ethyl ketone Test solution: Injection under examination. Heat the plate at 110° for 10 minutes. Use a plate such that during development the mobile phase migrates over a distance of 10 cm to 15 cm in about 10 minutes. (a) Apply to the plate 5 µl to 10 µl of the injection under examination. Allow the mobile phase to rise 10 cm to 15 cm and dry the plate in air. Determine the distribution of radioactivity using a suitable detector. Impurities in colloidal form remain at the starting point. Technetium pentetate complex and pertechnetate ion migrate near to the solvent front. (b) Apply to the plate 5 µl to 10 µl of the injection under examination. Allow the mobile phase to rise 10 cm to 15 cm and dry the plate. Determine the distribution of radioactivity using a suitable detector. Pertechnetate ion migrates near to the solvent front. Technetium pentetate complex and impurities in colloidal form remain at the starting point. The sum of the percentages of radioactivity corresponding to impurities in the chromatograms obtained in test (a) and (b) does not exceed 10.0 per cent. Method C Determine by Paper chromatography with Whatman No 1paper Solvents (a): 0.9 per cent w/v solution of sodium chloride in water. (b): Methyl ethyl ketone Spot about two to five microlitres of 99mTc- DTPA Injection with a capillary or a micropipette at the point of spotting marked at 3 cm from one end of the strip (3 X 23 cm) in duplicate. Develop the chromatogram in by ascending chromatography till the solvent front reaches 15 cm from the point of spotting.. Allow the strips to dry. Scan the strip using a radiochromatogram scanner with collimator suitable adjusted to measure 99mTc or cut into one cm sections and count in a well type NaI(Tl) scintillation counter. In PC saline, impurities in colloidal form remain at the starting point. Technetium DTPA complex and pertechnetate ion migrate near to the solvent front. In PC/saline, the radioactivity at the point of spotting gives the hydrolysed technetium whereas in PC/MEK , the pertechnetate moves to the solvent front. The sum of the percentages of radioactivity corresponding to impurities in the chromatograms obtained in test (a) and (b) does not exceed 10.0 per cent. Biodistribution. The whole body radioactivity retention shall not be more than 5% of the injected dose in 24 hours post injection in 2 out of 3 mice. Sterility (2.2.11). Complies with the test for sterility as described in general chapter on Radiopharmaceutical preparation. The injection may be released for use before completion of the test. Bacterial endotoxins Less than 175/V EU/mL V being the maximum recommended dose in mL. Storage. : Store at room temperature with adequate shielding. Use within 4h of time of preparation Labelling— The label states, in addition to the information specified under injections (1) the amount of 99mTc as labeled pentetic acid complex expressed as total megabecquerels (millicuries or microcuries) and concentration as megabecquerels (microcuries or millicuries) per ml at the time of calibration (2) the expiration date (3) the statement “Caution—Radioactive Material.”