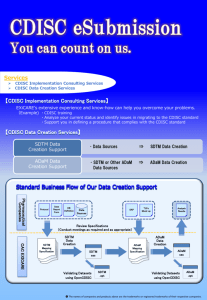
Eastern Research Group is working with FDA`s Office
advertisement

Eastern Research Group is working with FDA’s Office of the Commissioner to provide an economic analysis of the possible proposed regulation that will require electronic submission of clinical trial data using CDISC formats. Our objectives are to estimate the costs and possible cost savings to biopharmaceutical companies related to implementing and using CDISC standards. These estimates are required for any proposed regulation, in order to gauge whether the regulation will be a burden on the industry. The general questions we need to answer are: 1. How are companies planning to implement standards, and how long will implementation take? a. Are you already in process of implementing? why/why not? b. What type of implementation are you planning/carrying out? (e.g., collecting data in SDTM or near-SDTM formats; using a data repository to produce SDTM data; etc.) c. Are you implementing ADaM and define.xml as well as SDTM? d. How long do you anticipate your implementation will take? 2. What are the estimated total initial costs to implement standards for your company? 3. Do you anticipate incremental costs for the first few years? (e.g., do you expect that data handling will be more cumbersome for a while, or that costs will be higher for other reasons?) Please elaborate on the reasons and magnitude. 4. Do you expect cost savings once standards are in place? How soon? What level of savings do you anticipate? 5. How do you expect to address ongoing evolution of the standards? If you are involved with regional user groups and can answer in general for the group, that would be great, or you can pass the questions on to members of your group. If you’d like to discuss this further, I’ll be happy to set up a time to talk. Thank you! Marisa Mazzotta, Ph.D. Senior Economist Eastern Research Group 401-229-2389 mjmazzotta@cox.net