Elements, Compounds And The Atomic Theory
advertisement

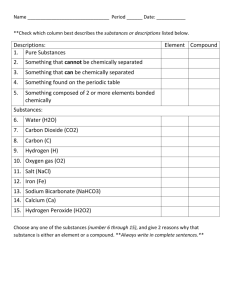
PURE SUBSTANCES There are millions of kinds of PURE SUBSTANCES. As scientists began studying substances, they found that there were certain simple substances that were in all of the materials that they studied. They called these "building blocks of matter" elements. Elements are pure substances that cannot be broken down to any simpler substance. Compounds, as we have seen, are substances made up of two or more elements combined in specific proportions. ex. If each letter in the alphabet is an element, then words would be compounds. Ex. Hydrogen + Oxygen = Water (H20) Carbon + Hydrogen + Oxygen = Alcohol There are only about 100 elements in the universe that we know. Each of these elements has their own specific properties. Some are more common, some rare, some poisonous, some radioactive, and some explosive. As scientists began studying substances, they found that there were certain simple substances that were in all of the materials that they studied. They called these "building blocks of matter" elements. Elements are pure substances that cannot be broken down to any simpler substance. Compounds, as we have seen, are substances made up of two or more elements combined in specific proportions. ex. If each letter in the alphabet is an element, then words would be compounds. Ex. Hydrogen + Oxygen = Water (H20) Carbon + Hydrogen + Oxygen = Alcohol Elements, Compounds And The Atomic Theory Atoms are the base of chemistry! They are the base for everything in the Universe! Matter is composed of atoms. The Particle Theory states that all matter is made of particles. The Atomic Theory goes farther to say that there is a difference between elements and compounds. Atoms are the smallest particles of elements. Since there are about 100 elements, there are about 100 kinds of atoms. Atoms can join together in many combinations to form molecules. If the atoms of a molecule are the same, the substance is an element. If the atoms of a molecule are different, the substance is a compound. Compounds and Their Proportions Water carbon dioxide carbon monoxide hydrogen peroxide All elements are represented by a chemical symbol. It is either a single capital letter, or a capital letter followed by a small letter. Examples: Ca = calcium Cu = copper C = carbon N = nitrogen Combinations of symbols represent compounds. These compounds are called chemical formulas. Example: H20 2 hydrogen atoms 1 oxygen atom If no number is shown beside the symbol, a 1 is understood. If more than 1 atom is present, a small number is shown after the atom. NaHCO3 = Sodium Hydrogen Carbonate (baking soda) 1 atom Na = Sodium 1 atom H = Hydrogen 1 atom C = Carbon 3 atoms O = Oxygen Elements are pure substances that cannot be broken down into any other substances by chemical or physical means. Elements make up all of the different kinds of matter in the universe either as single elements or in combination with other elements. The Periodic Table of elements is a way of organizing all known elements by their physical and chemical properties. An element is identified by its specific chemical and physical properties. Each element has a chemical symbol, which is usually one or two letters representing the element. If the symbol for an element is a single letter, then the letter is always capitalized. If the symbol is two letters, then the first letter is always capitalized and the second letter is lowercase. The symbols for each element are shown on the Periodic Table and are used to represent elements in chemical formulas and equations. Chemical symbols consist of letters of the Latin alphabet, although they are intended to be used by people of all languages. Examples of elements with symbols based on Latin names are copper (Cu- Cuprum), gold (Au – Aurum), and silver (Ag – Argentum). Although there are a little more than 100 different elements in the world, only a small number comprise a majority of the different systems on Earth. The elements that make up rocks and minerals of solid Earth include silicon, oxygen, iron, magnesium, and other trace amounts of various elements. Living organisms are composed primarily of carbon, oxygen, and hydrogen. Other elements in living matter include calcium, nitrogen, phosphorus, potassium, and trace amounts of other elements. Water is made up of hydrogen and oxygen. The oceans also contain salt, made up of sodium and chlorine. The atmosphere is mostly made up of the element nitrogen, existing in diatomic form (N2). It also includes oxygen, carbon (in carbon dioxide) and a small amount of other elements. Even though most of the other elements do occur naturally, they do so in very small amounts compared to the limited number of elements making up the majority of Earth’s systems. The most abundant elements on Earth are located within the first twenty elements on the Periodic Table. Elements consist of one type of atom distinguished by its atomic number which represents the number of protons in its nucleus. An atom that has a different number of protons represents a completely different element. Only a few elements exist in nature as pure elements. Examples include nitrogen gas (N2), oxygen gas (O2), gold (Au), carbon (C), silver (Ag), and copper (Cu). Many metals exist in pure element form. Most matter consists of elements combined with other elements in a set ratio or proportion called compounds. For example, water is a compound made of the elements hydrogen and oxygen. The ratio of these elements is two hydrogen atoms and one oxygen atom for each molecule of the compound water. Carbon dioxide gas is another example of a compound. Any sample of carbon dioxide is always made of part carbon to two parts oxygen (CO2). Salt, sodium chloride is made of one part sodium to one part chlorine (NaCl). When elements are combined to make a compound, the new substance has properties different from those of the original elements. For example, table salt is made of two elements, sodium and chlorine. The properties of table salt do not represent the properties of the individual elements. Sodium in element form is a highly reactive metal and chlorine is usually a yellow-green gas in its natural state. When combined to make salt, the properties of the compound are considerably different. http://kleinisd.stemscopes.com/text_elements/112 Substance Formula Chlorine Hydrogen Hydrochloric Acid Purified Water Sodium Chloride Carbon Dioxide Carbon Monoxide Magnesium powder Carbonic Acid Oxygen Cl H HCl H2O Salt NaCl CO2 CO Mg H2CO3 O Draw a model of an element: Draw a model of a compound: Draw a model of a mixture: Give some characteristics of elements: Element or Compound