Buffers - Mwiseman.com
advertisement

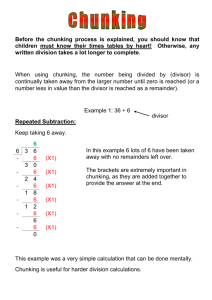
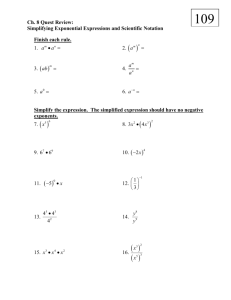
Buffers Buffers resist changes in pH. This happens as a result of the common ion effect and can be explained using equilibrium principles What is a buffer? o Mixture of a weak acid or base and a soluble salt made of the corresponding conjugate base or acid HF + NaF Hydrofluoric acid is a weak acid with a Ka of 7.2 x 10-4 HF H+ + F Give the above eqtn, according to LeChatelier, what will happen when additional Fluoride ion is added? Equilibrium will shift to the reactants, amount of H+ will be lower than if no excess Fluoride ion was added When there is excess fluoride ion, this solution can resist changes in pH Adding Base: When OH- is added, the H+ gets used up causing more of the weak acid to dissociate - maintaining the same pH. As long as the added amount of base does not exceed the initial concentration of weak acid. Adding Acid: When H+ is added the equilibrium shifts towards the reactants, HF. The excess fluoride in the solution bonds with the excess H+ to form HF such that the net H+ doesn’t change significantly and pH is maintained. The Math Steps for non-buffer systems 1. Write dissociation reaction 2. Write equilibrium expression 3. Write starting concentrations for acid 4. Concentrations of [H+] = [A-] = x and [HA] = [HA]0 – x 5. Plug into equilibrium expression and set equal to Ka 6. Make the assumption that for weak acids x is very small and, [HA]0 – x [HA]0 7. Solve for x, x = [H+] 8. Plug into pH = - log [H+] Non-buffer systems: 1. Calculate the pH of a solution given concentration and Ka a. 1.5 M Ethanoic acid, HC2H3O2 has a Ka = 1.8 x 10-5 mol dm-3 HC 2 H 3O2 H C 2 H 3O21 [ H ][C 2 H 3O21 ] Ka 1.8 x10 5 [ HC 2 H 3O] [ H ] [C 2 H 3O21 ] x [ HC 2 H 3O] 1.5 x 1.5 [ x][ x] x 2 1.5 x 1.5 x 2.7 x10 5 [ H ] 1.8 x10 5 pH log[ H ] log( 2.7 x10 5 ) 4.6 2. Calculate mass given desired pH and pKa a. Give the number of moles of benzoic acid must be dissolved in 100 cm 3 of water for a solution of pH 3.4? The pKa of benzoic acid is 4.20. i. Does it matter that you don’t know what benzoic acid is? No – b/c it’s an acid which means it donates a proton and the acid dissociation is the same as all the rest. We can substitute the actual formula of benzoic acid with HBz. HBz H Bz 1 pH 3.4 log[ H ] [ H ] 1x10 ( 3.4 ) 3.98 x10 4 M [ H ] [C 2 H 3O21 ] 3.98 x10 4 M pKa 4.20 Ka 1x10 ( 4.20) 6.3x10 5 Ka [ H ][ Bz 1 ] 6.3 x10 5 [ HBz ] 6.3 x10 5 [ H ]2 [ H ]2 (3.98 x10 4 M ) 2 x x x [H ] x 2.5 x10 3 M [ HBz ] x 0.100dm 3 x 2.5 x10 4 mols 2.5 x10 3 M Buffer systems 1. Calculate the pH of buffer system a. A buffer containing 0.20 mol of sodium ethanoate (NaC 2H5O2) in 500 cm 3 of 0.10 mol dm-3 ethanoic acid. Ka for ethanoic acid is 1.8 x 10-5. - assume complete dissociation of sodium ethanoate - assume the dissociation of ethanoic acid is insignificant such that the equilibrium concentration of ethanoic acid is the same as the initial concentration NaC2 H 3O2 Na C 2 H 3O21 HC 2 H 3O2 H C 2 H 3O21 Ka [ H ][C 2 H 3O21 ] 1.8 x10 5 [ HC 2 H 3O] [H ] x [C 2 H 3O21 ] (0.20mol ) /( 0.500dm 3 ) 0.40 M [ HC 2 H 3O] 0.1M x 0.1M [ x][0.40] [ x][0.40] 0.1 x 0.1 6 x 4.5 x10 [ H ] 1.8 x10 5 pH log[ H ] log( 4.5 x10 6 ) 5.3 b. Calculate the mass of sodium propanoate must be dissolved in 1.0 dm 3 of 1.0 mol/dm3 propanoic acid (pKa = 4.87) to give a buffer solution with a pH of 4.5. HC 3 H 5 O2 H C3 H 5 0 2 1 pH 4.5 log[ H ] [ H ] 1x10 ( 4.5) 3.16 x10 5 M 1 [C3 H 5 0 2 ] x [ HC 3 H 5 0 2 ] 1.0 M 3.16 x10 5 M 1.0 M pKa 4.87 Ka 1x10 ( 4.87) 1.35 x10 5 1 [ H ][C3 H 5 0 2 ] Ka 1.35 x10 5 [ HC 3 H 5 0 2 ] 1.35 x10 5 (3.16 x10 5 )( x) 1.0 x 0.427 M y 1.0dm 3 y .427 mols 0.427 M NaC3 H 5 0 2 96.07 g / mol 96.07 g / mol * 0.427 mol 41.0 g 2. Calculate the pH of a buffer after base is added a. A buffer containing 0.20 mol of sodium ethanoate (NaC 2H5O2) in 500 cm 3 of 0.10 mol dm-3 ethanoic acid. Ka for ethanoic acid is 1.8 x 10-5. 0.025 moles of sodium hydroxide is added. i. Hydroxide will cause the acid dissociation to shift to the right ii. So the amount of hydroxide added must be subtracted from the ethanoic acid and added to the amount of ethanoate NaC 2 H 3 O2 Na C 2 H 3 O21 HC 2 H 3 O2 H C 2 H 3 O21 Ka [ H ][C 2 H 3 O21 ] 1.8 x10 5 [ HC 2 H 3 O] [H ] x [C 2 H 3 O21 ] (0.20mol 0.025mol ) /( 0.500dm 3 ) 0.45M [ HC 2 H 3 O] [0.1M (0.025) /( 0.500dm 3 )] x 0.05M x 0.05M [ x][0.45] [ x][0.45] 0.05 x 0.05 6 x 2.0 x10 [ H ] 1.8 x10 5 pH log[ H ] log( 2.0 x10 6 ) 5.7 3. Calculate the pH of a buffer after acid is added a. A buffer containing 0.20 mol of sodium ethanoate (NaC 2H5O2) in 500 cm 3 of 0.10 mol dm-3 ethanoic acid. Ka for ethanoic acid is 1.8 x 10-5. 0.025 moles of hydrochloric acid is added. i. Hydrogen ions will cause the acid dissociation to shift to the left ii. So the amount of hydrogn added must be added to the ethanoic acid and subtracted from the amount of ethanoate 4. NaC2 H 3O2 Na C 2 H 3O21 HC 2 H 3O2 H C 2 H 3O21 Ka [ H ][C 2 H 3O21 ] 1.8 x10 5 [ HC 2 H 3O] [H ] x [C 2 H 3O21 ] (0.20mol 0.025mol ) /( 0.500dm 3 ) 0.35M [ HC 2 H 3O] [0.1M (0.025) /( 0.500dm 3 )] x 0.15M x 0.15M [ x][0.35] [ x][0.35] 0.15 x 0.15 6 x 7.7 x10 [ H ] 1.8 x10 5 pH log[ H ] log( 7.7 x10 6 ) 5.1