click - Uplift Education
advertisement

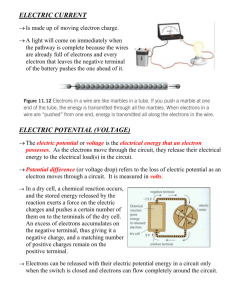
1 ad All physics to date has led to one primary conclusion: There are four fundamental forces: 1) Gravitational 2) Electromagnetic all based on the 3) Strong nuclear Electromagnetic Theory 4) Weak nuclear GUT - grand unified theory ~250 yrs or so since we first learned what electricity is “Electricity” – from the Greek word electron () - “amber”. The ancients knew that if you rub an amber rod with a piece of cloth, it attracts small pieces of leaves or dust. “amber effect”– the object becomes electrically charged Electric forces charges exert electric forces on other charges ◊ two positive charges repel each other ◊ two negative charges repel each other ◊ a positive and negative charge attract each other • The repulsive electric force between 2 protons is 1,000,000,000,000,000,000,000,000,000,000,000,000 times stronger than the attractive gravitational force! • Attractive force between protons and electrons cause them to form atoms. Electrical force is behind all of how atoms bond … chemistry • charge is measured in Coulombs [C] Electricity & Magnetism • static electricity (Electrostatics) ◊ Why do I get a shock when I walk across the rug and touch the door knob? ◊ Why do socks stick to my pants in the dryer? ◊ Why does my hair stick to my comb, and I hear a crackling sound ? ◊ Why does a piece of plastic refuse to leave my hand when I peel it off a package? ◊ What is lightning? • What is that all about? It’s the CHARGE No one has ever seen electric charge; it has no weight, color, smell, flavor, length, or width. Charge is an intrinsic property of matter – electron has it, proton has it, neutron doesn’t have it – and that’s all • Electric charge is defined by the effect (force) it produces. • Matter (stuff) has two basic properties ◊ mass is what gives the gravitational force ◊ charge is wh at gives electric and magnetic forces There are two kinds of charge: ◊ positive charge ◊ negative charge Named by Benjamin Franklin (1706 - 1790, American statesman, philosopher and scientist) Electricity has origin within the atom itself. French physicist Charles A. de Coulomb 1736 - 1806 -19 ◊ every electron has charge -1.6 x 10 C, -19 ◊ every proton 1.6 x 10 C 18 6.25 billion billion (6.25 x 10 ) electrons has charge of 1 C ! Yet 1C is the amount of charge passing through a 100-W light bulb in just over a second. A lot of electrons! let e 1.6 1019 C • The smallest amount of the free positive charge is the charge on the proton. q = e proton • The smallest amount of the free negative charge is the charge on the electron. q =-e electron • quarks have 1/3, but they come in triplets Charge is quantized: cannot divide up charge into smaller units than that of electron (or proton) i.e. all objects have a charge that is a whole-number multiple of charge of the smallest amount (a single e). • The net charge is the algebraic sum of the individual charges (+ 5 - 3 = 2). • Everyday objects - electronically neutral – balance of charge – no net charge. • Objects can be charged – there can be net charge on an object. How? The only type of charge that can move around is the negative charge, or electrons. The positive charge stays in the nuclei. So, we can put a NET CHARGE on different objects in two ways ◊ Add electrons and make the object negatively charged. ◊ Remove electrons and make the object positively charged. mnucleon 2000 melectron ratom 100000 rnucleus Atom is electrically neutral = has no net charge, since it contains equal numbers of protons and electrons. Some materials have atoms that have outer electrons (farthest from nucleus) loosely bound. They can be attracted and can actually move into an outer orbit of another type of atom. The atom that has lost an electron has a net charge +e (positive ion). An atom that gains an extra electron has a net charge of – e (negative ion). This type of charge transfer often occurs when two different materials (different types of atoms) come into contact. 2 • Which object gains the electrons depends on their electron affinity So, electrons can be transferred from one object to another During that process, the net charge produced is zero. The charges are separated, but the sum is zero. The amount of charge in the universe remains constant (we think!!) It is CONSERVED! • Electrons in insulators are tightly bound to atomic nuclei and so cannot be easily made to drift from one atom to the next. Only if a very strong electric field is applied, the breakthrough (molecules become ionized resulting in a flow of freed electrons) could result in destruction of the material. Most things are in between perfect conductor/ insulator Law of Conservation of charge: Charge is always conserved: charge cannot be created or destroyed, but can be transferred from one object to another. When objects are charged by rubbing, they don’t stay charged for ever. They eventually return to neutral state – very often the charge will “leak off” onto water (polar) molecules in the air. Sometimes they will be neutralized by charged ions in the air (formed, for example, by collisions with charged particles known as cosmic rays). Given enough time, the particles in the air will remove the excess charge from the object leaving it neutrally charged. This explains why on dry days we tend to have more trouble with static electricity build-up than on humid (moist) days. On moist days there are more water molecules in the air to steal charge more rapidly. On dry days there are fewer particles in the air to steal charges so we accumulate charge until we touch something and get discharged (shocked). Electrical conductors, insulators, semiconductors and superconductors - distinction based on their ability to conduct or transmit electric charge. Conductor: Any material that contains movable charges and allows charges to move about more or less freely. So, if you transfer some electrons to the metal rod, that excess of charge will distribute itself all around rod. Tap water, human body and metals are generally good conductors. Insulator: is a a material that doesn’t contain movable charges and resists the flow of electric current Materials like amber, teflon, glass, pure water, plastic, glass, rubber, wood are good insulators. ELECTROSTATIC CHARGING 1. Charging by Friction: The transfer of charge is due to the rubbing – friction between two previously neutral materials. When you move your comb through your hair, the friction (rubbing) between the comb and hair can pull some of the electrons out of your hair and onto the comb. As a result your comb ends up with a net negative charge and attracts your hair which is now positive. Rubbing: rubber rod with fur or cloth, glass rod with silk, hair with balloon, shuffling across a carpeted floor. 2. Charging by Conduction (Contact): 2.1 Conductors: When a charge is placed on a conductor, the mutual repulsion of the individual charges causes them to move as far away from each other as possible. Thus, a charge deposited on a conductor quickly spreads out over its surface. Metal sphere on insulating stand 2. 2 Insulators: When a charge is placed on an insulator, it remains where it is deposited and surrounding molecules become polarized. An external (negative) charge distorts the shape of an atom by forcing its negatively-charged electron clouds to shift away from the charge and the positively charged nuclei to shift toward the charge. Such a distorted atom is said to be polarized. That’s all very nice, but why is that so? What makes conductors conduct? • Atoms have equal numbers of positive and negative charges, so that a chunk of stuff usually has no net charge the plusses and minuses cancel each other. • However, in metal atoms the valence electrons – the electrons in the outermost orbits - are loosely bound, so when you put a bunch of metal atoms together (to form a metal) an amazing thing happens: valence electrons from each atom get confused and forget which atom they belong to. • They now belong to the metal as the whole. As the result, positive ions which are tightly bound and can only oscillate around their equilibrium positions, form a positive background. All the homeless electrons - “Free electrons”wander around freely keeping ions from falling apart – metallic bond!! Question: Consider a negatively charged rod touching a conductor versus an insulator. What is the difference between how the electrons are arranged on the conductor and insulator? • charges can be transferred from/to conductors or non-conductors but they can only move through conductors. Would spread out evenly on a good conductor, because the transferred electrons repel each other. But on insulator would be localized at where the rod touched. 3 3. Charging by Induction Example:: A charged rod is brought close to two metal spheres touching each other. Free electrons: since free to move, they will! 3.1 Conductors: Steps: 1. Neutral conductor with free electrons 2. free electrons in the metal are repelled as far as possible from the charged object. Charge has been separated, but metal sphere is still neutral. 3.. The Earth is reservoir of any charge. It can easily accept or give up electrons. Connect conductor with a conducting wire to the ground - many of free electrons in metal are able to move even further from charged object down the wire into the Earth. Or you can touch it with finger, electrons flow through your finger, through you, to the ground. 4. Object is left positively charged. 5. cut the wire, remove the rod and the metal sphere has evenly distributed positive charge. Once separated from each other with rod still close they’ll remain charged. Charge is conserved, so charges on spheres A and B are equal and opposite. Question: A metal ring receives a positive charge by contact. What happens to the mass of the ring? Does it increase, stay the same, or decrease? When the positively charged ball touches the ring, electrons inside it are attracted to the ball. Some will leave the ring trying to neutralize the ball. Only a tiny fraction leaves the ring. The mass of the electrons is so small compared to the atoms, so although the mass of the ring decreases, measuring it would not be possible. (By the way, both will be positively charged, but the ball will be less then before) 3.2 Insulators: When insulator is charged by induction, there will be no change of charge. Instead of that, charge within the molecule/atom move slightly in opposite directions (the net charge is kept zero) Therefore we call it rather: Positive Charging by Polarization surface charge A charge placed near an insulator polarizes its atoms. While the insulator’s interior remains electrically neutral, a net charge appears on the surface, and can produce force on other charges near the insulator. Even though sphere is neutral there is attraction force acting between the rod and sphere. What is polarization? When a charged object is brought near an insulator, electrons are not free to migrate throughout material. Instead, they redistribute within the atoms/molecules themselves: “centers of charge” move. Example: Van de Graaff The sphere gives the girl a large negative charge. Each strand of hair is trying to: Get away from the other strands of hair. Like charges attached to the hair strands repel, causing them to get away from each other. Example: electroscope the electroscope is a simple device for observing the presence of electric charge it consists of a small piece of metal foil (gold if possible) suspended from a rod with a metal ball at its top If a positively charged rod is placed near the ball, free electrons move closer to it leaving leaves positively charged.The two sides of the metal foil then separate. Example: Attracting uncharged objects In an usual atom center of electron cloud is at the center of positive nucleus When a negative charge is brought near the right, electron cloud shifts to the left. Centers of positive and negative charges no longer coincide. uncharged metal sphere A negatively charged rod will push the electrons to the far side leaving the near side positive. The force is attractive because the positive charges are closer to the rod than the negative charges 4 Example: Charge polarization is why a charged object can attract a neutral one : DEMO: Rub balloon on your hair – it will then stick to the wall ! Why? Balloon becomes charged by friction when rubbed on hair, picking up electrons. It then polarizes molecules on the surface, inducing + charge layer on the wall’s surface closest to it , and next layer negative furthest away. So balloon is attracted to + charges and repelled by – charges in wall , but the – charges are further away so repulsive force is weaker and attraction wins. Example: You can bend water with charge! The water molecule has a positive end and a negative end. When a negative rod is brought near the stream of water, all the positive ends of the water molecules turn to the right and are attracted to the negative rod. What happens if the rod is charged positively? • As we said like charges repel, and opposite charges attract. • This is the fundamental cause of almost ALL electromagnetic behavior. • But how much? • How Strong is the Electric Force between two charges? a) They exert equal forces on each other only in opposite direction F k q1q2 0.40 N r2 (“-“ = attractive force) b) r = 3 m F k q1q2 1.6 N 4 F r2 At very small separation - spark -5 How many electrons is 2.0 x 10 C ? 2.0 10 5 C 10 14 electrons 1.6 10 19 C Trivia: When you comb your hair with a plastic comb, some electrons from your hair can jump onto it making it negatively charged. 28 Your body contains more than 10 electrons. Suppose that you could borrow all the electrons from a friend’s body and put them into your pocket. The mass of electrons would be about 10 grams (a small sweet). With no electrons your friend would have a huge positive charge. You, on the other hand, would have a huge negative charge in your pocket. If you stood 10 m from your friend the attractive force would be 23 equal to the force that 10 tons would exert sitting on your shoulders – more 100,000 times greater than the gravitational force between the earth and the Sun. Luckily only smaller charge imbalances occur, so huge electrical forces like the one described simply do not occur. Example: Three point charges : ELECTROSTATIC – ELECTRIC - COULOMB FORCE The force between two point charges is proportional to the product of the amount of the charge on each one, and inversely proportional to the square of the distance between them. F k q1q2 r2 q1= +8.00 mC; q2= -5.00 mC and q3= +5.00 mC. (a) Determine the net force (magnitude and direction) exerted on q1 by the other two charges. (b) If q1 had a mass of 1.50 g and it were free to move, what would be its acceleration? Force diagram k 8.99 109 N m 2 / C 2 Force is a vector, therefore it must always have a direction. F2 = k Question: -5 SHE accumulates charge q = 2.0 x 10 C 1 sliding out of the seat of a car. -5 HE accumulates charge q = -8.0 x 10 C 2 while waiting in the wind. What is the force between them a) when she opens the door 6.0 m from him and b) when their separation is reduced by half to 3 m? q1q2 = 0.213N r2 F3 = k q1q3 = 0.213N r2 x-components will cancel, because of the symmetry F = 0.213 sin230 + 0.213 sin230 = 0.166 N . a F 0166 m / s2 m 1.5 103 a = 111 m/s2 5 Electric Field Let's take a single electric charge, Q, and put it somewhere. The space around it is different from the space without charge. We have created a situation in which we could have an electric force. All we have to do is bring in a second charge, q, to feel the force. Without q, there is no force ....but we still have the condition that we could have a force. We say that the space around charge contains ELECTRIC FIELD. Example: Say the electric field from an isolated point charge has a certain value at a distance of 1m. How will the electric field strength compare at a distance of 2 m from the charge? It will be ¼ as much – inverse square law for force between two charges carries over to the electric field from a point charge. Electric field lines How to measure/find the strength (magnitude and direction) of electric field at particular location P due to charge Q? We use “Electric Field Lines” to visualize el. field. Convention / agreement A test charge, q, placed at P will experience an electric force, F either attractive or repulsive. Instead of drawing vector E at every point around certain charge (a huge mess) we decided to go with direction of electric field and density of electric field lines indicating the magnitude of el. field. Definition of electric field, E, at a point P distance r away from Q. The magnitude of the electric field is defined as the force per unit charge. E= F q E positive charge negative charge N C As F contains q, E DOESN’T depend on q at all, only on Q. Electric field at any point P in space is always in the direction of the force on a positive test charge if it were placed at the point P. Denser lines - stronger field → el. field decreases with distance The other way around: If you know electric field E at a point where you place a charge q , that charge will experience the force F: F=Eq more lines revels stronger field due to greater charge Electric field of a charged particle/point charge ◊ A charged particle Q creates an electric field. ◊ magnitude E= F Q =k 2 q r E Field independent of test charge the same value on the sphere of radius r around charge q ◊ direction – radially outward or inward. Example: q (test charge) E 9 109 1.6 1019 10 10 2 2.9 1011 N / C to the right El field always point away from + charges, towards – charge Electric field lines can never cross. If they crossed, that would mean that a charge placed at the intersection, would be accelerated in TWO directions at once! This is impossible! If two sources are creating electric fields in the same place, we have to add the two vectors and get a resultant vector representing the NET ELECTRIC FIELD. Example: What is the direction of the electric field at point C if the two charges have equal magnitude?? 6 Example: What is the direction of the electric field at point A if the two positive charges have equal magnitude? Example: if not then electrons would respond to its presence and be accelerated within the conductor. And that is not conductor in electrostatic equilibrium. Q.E.D If such conductor has excess charge, it resides entirely on the conductor’s outer surface running away from each other as far as possible. El. field is still zero everywhere inside the conductor. Therefore, electric field of charged conducting sphere in electrostatic equilibrium is: What is the direction of the electric field at point B if the two positive charges have equal magnitude?? Example: What is the direction of the electric field at point A, if the two positive charges have equal magnitude? Electric field of a capacitor Uniform electric field (the one that has constant magnitude and direction is generated between two oppositely charged parallel plates. Edge effect is minimized when the length is long compared to their separation. El. field just outside a charged conductor is perpendicular to the conductor’s surface. Electric field of a charged conducting sphere in electrostatic equilibrium ◊ Electric field outside a charged sphere (evenly distributed charge q over surface) at distance r from its center is the same as if the charge q is concentrated at the center of the sphere: if not ◊ What is electric field inside the sphere? OK. Let’s start. In general if you have many charges you have to find el. field of each of them and then add them up as vectors to get net el. field at certain point. So imagine a solid!!!!!!!!! Good luck. Charge tends to accumulate where the curvature is greatest (sharp points), resulting in strongest electric field. ◊ Different approach: Conductor is in electrostatic equilibrium when there is no net motion (flow ) of charge within a conductor or on its surface. • Conductor electrons free to move • charges in electric field feel the force F = Eq • only free electrons can move in the conductor so they will move until E = 0 inside a conductor The fact that pointed objects create strong electric fields if 7 charged is the reason for the shape of lightning rod. Electrical Energy and Electrical Potential Two different things that sound alike! Recall Work: W = F d cos(θ) • In order to bring two like charges together work must be done. • In order to separate two opposite charges, work must be done. • This work done by external force against electrical force is stored as electrical PE, U. • If released , charges will gain KE while losing potential energy U . • So essentially, potential energy is capacity for doing work which arises from position or configuration. • Greater amount of charge → greater force needed → greater work done → greater stored potential energy U. • → introducing the electrical potential energy per unit charge, known as electrical potential, which does not depend on the amount of charge. Electric potential If a charge q at point P (in electric field E) has electric potential energy U, the electric potential V at that point is: electric potential V electric potential energy charge U q ◊ The SI unit of electric potential is the volt: 1V 1J 1C • Because the dependence on the charge q has been divided out, the electric potential depends only on position. V1 < V2 V1 > V2 Positive charge accelerates from higher to lower potential. • Note important difference between energy and potential: • A point has potential, charge placed there has electric potential energy • Two points that are at the same distance from the charged object have the same potential. • So, when two charged object are placed there, they are at the same potential, but the one with more charge on it has higher electric potential energy. Potential Difference Between Two Points (ΔV = VB – VA) The amount of gravitational potential energy depends on the reference point. In contrary, here we choose that: . Law of conservation of energy: change in potential energy = change in kinetic energy The zero of electrical potential and potential energy is at infinity. Work – kinetic energy theorem work done by net force (electric force) = change in kinetic energy Negative charge accelerates from lower to higher potential. The difference between the potentials at two different points (A & B) measures the work done per unit positive charge in order to move it from one point to the other. V W U q q So now we reformulate definition of electric potential: The potential, V at a point P in an electric field, is the work done per unit of positive charge in order to bring it from infinity to that point. The zero of electrical potential is at infinity. Point charge Q: Potential energy of charge q at point P r distance from charge Q: Q Qq and potential at point P is: U=k V k r r Electrical potentials can be positive or negative. → If a charge, q, is moved through a potential difference, ∆V, then the work done on it is equal to the change in its electric potential energy which is converted into kinetic energy: W = ∆U = q ∆V = ½ mv2 electron-Volt (eV) An electron volt is the amount of energy/work it takes to move an electron through a potential difference of 1volt. 1 eV = ∆U = W = (1.6x10-19 C) (1V) 1 eV = 1.6x10-19 J The electron volt is not a smaller unit for volts!!! 8 Electrical resistance (symbol R) It is a smaller unit for energy. Uniform electric field, E Why is it necessary to keep pushing the charges to make them move? The electrons do not move unimpeded through a conductor. As they move they keep bumping into the ions of crystal lattice which either slows them down or bring them to rest. A ball accelerates as it loses potential energy. Similarly, a positive charge accelerates from a region of higher potential toward a region of lower potential. The resistance (R) is a measure of the degree to which the conductor impedes the flow of current. Resistance is measured in Ohms () OHM’S LAW - Current, Voltage and Resistance ◊ The change in potential energy of a charge q is converted into kinetic energy: ∆U = q ∆V = ½ mv2 ◊ The change in kinetic energy is equal to the work done on charge q by el. field: Fd = qEd = ½ mv2 ◊ relationship between uniform electric field E and potential difference between two points distance d from each other along electric field line: ΔV = Ed → E = ΔV/d and NC-1 = Vm-1 Electric current (symbol I) ◊ the flow of electric charge q that can occur in solids, liquids and gases. DEF: the rate at which charge flows past a given cross-section. I= measured in amperes (A) DEF: Current through resistor (conductor) is proportional to potential difference on the resistor if the temperature of a resistor is constant (the resistance of a conductor is constant). math def: I= V R if resistance R is constant/ temperature is constant I – current V – potential difference across R Example: If a 3 volt flashlight bulb has a resistance of 9 ohms, how much current will it draw? I = V / R = 3 V / 9 = 1/3 Amps If a light bulb draws 2 A of current when connected to a 120 volt circuit, what is the resistance of the light bulb? R = V / I = 120 V / 2 A = 60 Factors affecting resistance Conductors, semiconductors and insulators differ in their resistance to current flow. DEF: The electrical resistance of a piece of material is defined by the ratio of the potential difference across the material to the current that flows through it. R= q t 1C 1s Solids – electrons in metals and graphite, and holes in semiconductors Liquids – positive and negative ions in molten and aqueous electrolytes Gases – electrons and positive ions stripped from gaseous molecules by large potential differences. 1A = V I The units of resistance are volts per ampere (VA-1). However, a separate SI unit called the ohm Ω is defined as the resistance through which a current of 1 A flows when a potential difference of 1 V is applied. Wires, wires, wires As you are going to see, the resistance of a wire can be completely ignored – if it is a thin wire connecting two, three or more resistors, or becoming very important if it is a long, long wire as in the case of iron, washing machine, toaster ….., where it becomes resistor itself. 9 The resistance of a conducting wire depends on four main factors: • length • cross-sectional area • resistivity • temperature • Cross Sectional Area (A) r = 1.00 mm ρ = 1.72x10-8 Ωm, copper - books R = r L/A = (1.72x10-8 )(1.67)/(7.85x10-7) = 3.50 Ω I = V / R = 1.5 / 3.5 = 0.428 A The cross-sectional area of a conductor (thickness) is similar to the cross section of a hallway. If the hall is very wide, it will allow a high current through it, while a narrow hall would be difficult to get through. Notice that the electrons seem to be moving at the same speed in each one but there are many more electrons in the larger wire. This results in a larger current which leads us to say that the resistance is less in a wire with a larger cross sectional area. Ohmic and Non-Ohmic conductors How does the current varies with potential difference for some typical devices? metal at const. temp. filament lamp diode • Length of the Conductor (L) The length of a conductor is similar to the length of a hallway. A shorter hallway will result in less collisions than a longer one. I = 1 is const. R is const. V R • Temperature To understand the effect of temperature you must picture what happens in a conductor as it is heated. Heat on the atomic or molecular scale is a direct representation of the vibration of the atoms or molecules. Higher temperature means more vibrations. In a cold wire ions in crystal lattice are not vibrating much so the electrons can run between them fairly rapidly. As the conductor heats up, the ions start vibrating. As their motion becomes more erratic they are more likely to get in the way and disrupt the flow of the electrons. As a result, the higher the temperature, the higher the resistance. At extremely low temperatures, some materials, known as superconductors, have no measurable resistance. This is called superconductivity. Gradually, we are creating materials that become superconductors at higher temperatures and the race is on to find or create materials that superconduct at room temperature. We are painfully far away from the finish line. • Material used - resistivity The resistivity, ρ (the Greek letter rho), is a value that only depends on the material being used. It is tabulated and you can find it in the books. For example, gold would have a lower value than lead or zinc, because it is a better conductor than they are. The unit is Ω•m. Resistance of a wire when the temperature is kept constant is: R=ρ L A • In conclusion, we could say that a short fat cold wire makes the best conductor. • If you double the length of a wire, you will double the resistance of the wire. • If you double the cross sectional area of a wire you will cut its resistance in half. Example A copper wire has a length of 160 m and a diameter of 1.00 mm. If the wire is connected to a 1.5-volt battery, how much current flows through the wire? L = 1.60 m. Devices for which current through them is directly proportional to the potential difference across device are said to be ‘ohmic devices’ or ‘ohmic conductors’ or simply resistors. There are very few devices that are truly ohmic. However, many useful devices obey the law at least over a reasonable range. Devices are non-ohmic if resistance changes Power dissipation in resistors DEF: Electric power is the rate at which energy is supplied to or used by a device. DEF: Power is the rate at which electric energy is converted into another form such as mechanical energy, heat, or light. When a current is flowing through a load such as a resistor, it dissipates energy in it. In collision with lattice ions electrons’ kinetic energy is transferred to the ions, and as a result the amplitude of vibrations of the ions increases and therefore the temperature of the device increases. That thermal energy (internal energy) is then transferred as heat (to the air, food, hair etc.) by convection, or radiated as light (electric bulb). Where is that energy coming from? This energy is equal to the potential energy lost by the charge as it moves through the potential difference that exists between the terminals of the load. • Power is measured in J s-1 called watts W. If a vacuum cleaner has a power rating of 500 W, it means it is converting electrical energy to mechanical, sound and heat energy at the rate of 500 J s-1. A 60 W light globe converts electrical energy to light and heat energy at the rate of 60 J s -1. Deriving expressions for determining power Basic definition of power: P= Remember: W = qV → P = qV/t P = IV W t and I = q/t, so 10 2 2 P = V /R = I R 1J 1W = = 1A 1V 1s In USA you can not get direct information on power of appliance you buy. Look at your hair dryer. If label says “10 A”, that means that the power of the hair dryer is 10x120=1200 W, or 1.2 kW (using a standard US 120 V outlet). Comparison of US and other countries that use voltage of 240 V. As the power of appliances is the roughly the same, the appliances in USA have to draw a greater current, hence have to have less resistance. As the consequence the wires (both used for connecting and in appliances) are thicker in USA. example How much current is drawn by a 60 Watt light bulb connected to a 120 V power line? P = 60 W = I V = I x 120 so I = 0.5 A What is the resistance of the bulb? I = V/R R = V/I = 120 V/0.5 A R = 240 Paying for electricity You pay for the total amount of electrical energy (not power) that is used each month In Irving the cost of electric energy used is 14 ¢ per kilowatt-hour. How do we get kilowatt-hour and what is that? Power = energy/time Energy = power x time, so energy can be expressed in units watts x second what is simply a joule. Physicists measure energy in joules, but utility companies customarily charge energy in units of kilowatt-hours (kW h), where Kilowatt-hour (kWh) = 103 W x 3600 s 1W x 1s = 1J 1 kWh = 3.6 x 106 J $$$ example $$$ At a rate of 14 cents per kWh, how much does it cost to keep a 100 W light bulb on for one day? DEF: The electrons go one way but the current flows the opposite to the direction that the electrons travel. That’s convention. Drift speed When a battery is connected across the ends of a metal wire, an electric field is produced in the wire. All free electrons in the circuit start moving at the same time. Free electrons are accelerated along their path reaching enormous speeds of about 106 ms-1. They collide with positive ions of crystal lattice generating heat that causes the temperature of the metal to increse. After this event, they are again accelerated because of the electric field, until the next collision occurs. Due to the collisions with positive ions of crystal lattice, hence changing direction, it is estimated that the drift velocity is only a small fraction of a metre each second (about 10-4 m s-1). example: in an el. circuit of a car, electrons have average drift speed of about 0.01 cm/s, so it takes ~ 3 hour for an electron to travel through 1m. it’s not even a snail’s pace!!!!! The electricity that you get from the power company is not DC it is AC (alternating) created by an AC electric generator. In an AC circuit the current reverses direction periodically The current in AC electricity alternates in direction. The back-andforth motion occurs at freq. of 50 or 60 Hz, depending on the electrical system of the country. !!!!!!! the source of electrons is wire itself – free electrons in it !!!!!! If you are jolted by electric shock, electrons making up the current in your body originate in your body. They do NOT come from the wire through your body into the ground. Alternating electric field causes electrons to vibrate. Small vibrations – tingle; large vibrations can be fatal. • How does the voltage and current change in time? energy (kWh) = power (kW) x time (h) DC does not change direction over time; energy (kWh) = 0.1 kW x 24 h = 2.4 kWh cost / day = 2.4 kWh x 14 cents/kWh = 33.6 ¢ the actual voltage in a 120-V AC circuit varies between +170V and -170V peaks. for one month that amounts to $ 10.1. Direct Current (DC) electric circuits a circuit containing a battery is a DC circuit in a DC circuit the current always flows in the same direction. The direction of the current depends on how you connect the battery Either way the bulb will be on. a circuit must provide a closed path for the current to circulate around when the electrons pass through the light bulb they loose some of their energy the conductor (resistor) heats up the battery is like a pump that re-energizes them each time they pass through it Electromotive force (emf – ε or E) We have defined potential difference as the amount of work that has to be done to move a unit positive charge from one point to the other in an electric field. ΔV = W ΔU = q q A battery or an electric generator that transforms one type of energy into electric energy is called source of electromotive force 11 DEF: emf (ε) of the source is the potential difference between the terminals when NO current flows to an external circuit. IT IS A VOLTAGE NOT A FORCE. In the true sense, electromotive force (emf) is the work (energy) per unit charge made available by an electrical source. 2. loop rule – conservation of energy principle: Energy supplied equals the energy released in this closed path In a closed loop, the sum of the emfs equals the sum of the potential drops V = V 1 + V2 + V3 Resistors in Series D.C. circuit analysis Electric Circuits: Any path along which electrons can flow is a circuit. For a continuous flow of electrons, there must be a complete circuit with no gaps. A gap is usually provided by an electric switch that can be opened or closed to either cut off or allow electron flow. An electric circuit has three essential components • connected in such a way that all components have the same current through hem. • Burning out of one of the lamp filaments or simply opening the switch could cause such a break. Equivalent or total or effective or resistance is the one that could replace all resistors resulting in the same current. 1. A source of emf. 2. A conducting pathway obtained by conducting wires or some alternative. 3. A load to consume energy such as a filament globe, other resistors and electronic components. When the switch is closed, a current exists almost immediately in all circuit. The current does not “pile up” anywhere but flows through the whole circuit. Electrons in all circuit begin to move at once. Eventually the electrons move all the way around the circuit. A break anywhere in the path results in an open circuit, and the flow of electrons ceases. Req = R1+ R2 + R3 logic: the total or effective resistance would have length L1+ L2+ L3 and resistance is proportional to the length Terminal voltage, emf and internal resistance Resistors in Parallel In the circuit the total energy supplied is determined by the value of the emf. When electrons flow around a circuit, they gain potential energy in the cell and then lose the energy in the resistors. In a closed circuits charge must flow between the electrodes of the battery and there is always some hindrance to completely free flow. So when the current I is drawn from the battery there is some resistance called INTERNAL RESISTANCE (r ) of the battery causing the voltage between terminals to drop below the maximum value specified by the battery’s emf. • Electric devices connected in parallel are connected to the same two points of an electric circuit, so all components have the same potential difference across them. • The current flowing into the point of splitting is equal to the sum of the currents flowing out at that point: I = I1 + I2 + I3. Thus the TERMINAL VOLTAGE (the actual voltage delivered) is: V = e - Ir In the mid-nineteenth century, G.R. Kirchoff (1824-1887) stated two simple rules using the laws of conservation of energy and charge to help in the analysis of direct current circuits. These rules are called Kirchoff’s rules. 1. Junction rule – conservation of charge. The sum of the currents flowing into a point in a circuit equals the sum of the currents flowing out at that point. I1 + I 2 = I 3 + I 4 + I 5 1 1 1 1 = + + Req R1 R2 R3 • A break in any one path does not interrupt the flow of charge in the other paths. Each device operates independently of the other devices. The greater resistance, the smaller current. 12 example: Find power of the source, current in each resistor, terminal potential, potential drop across each resistor and power dissipated in each resistor. Req = 120 Ω I = ε ∕ Req = 0.3 A terminal potential: V = ε – Ir = 36 – 0.3x6.7 = 34 V current through resistors 100Ω and 50Ω : I = I1 + I2 0.3 = I1 + I2 Resistors in compound circuits 100 I1 = 50 I2 → I1 = 0.1 A I1R1 = I2R2 I2 = 0.2 A potential drops V = IR power dissipated P = IV 80 Ω 0.3x80 = 24 V 0.3x24 = 7.2 W 100 Ω 0.1x100 = 10 V 0.1x10 = 1 W 50 Ω 0.2x50 = 10 V 0.2x10 = 2 W 6.7 Ω 0.3x6.7 = 2 V 0.3x2 = 0.6 W ε = Σ all potential drops: 36 V = 2 V + 24 V + 10 V power dissipated in the circuit = power of the source 0.6 + 2 + 1 + 7.2 = 0.3x36 Ammeters and voltmeters In practical use, we need to be able to measure currents through components and voltages across various components in electrical circuits. To do this, we use AMMETERS and VOLTMETERS. An ammeter – measures current passing through it • is always connected in series with a component we want to measure in order that whatever current passes through the component also passes the ammeter. Now you can calculate current, potential drop and power dissipated through each resistor • has a very low resistance compared with the resistance of the circuit so that it will not alter the current the current being measured. • would ideally have no resistance with no potential difference across it so no energy would be dissipated in it. 13 A potential divider In electronic systems, it is often necessary to obtain smaller voltages from larger voltages for the various electronic circuits. A potential divider is a device that produces the required voltage for a component from a larger voltage. It consists of a series of resistors or a rheostat (variable resistor) connected in series in a circuit. A voltmeter – measures voltage drop between two points • is always connected across a device (in parallel). • has a very high resistance so that it takes very little current from the device whose potential difference is being measured. • an ideal voltmeter would have infinite resistance with no current passing through it and no energy would be dissipated in it. Potential divider equation I= V R1+R 2 V1 = V1 = IR1 R1 V R1+R2 example: In the potential divider shown, calculate: (a) the total current in the circuit (b) the potential difference across each resistor (c) the voltmeter reading if it was connected between terminals 2 and 6. (a) The total resistance R = 12 Ω. I = V / R = 12 V / 12 Ω = 1 A (b) 6 x V = 12 V → V = 2 V (12 V is equally shared by each 2 Ω resistor. or V = IR = 1x2 = 2 V (c) R = 4 x 2 = 8 Ω Between terminals 2 and 6 there are 4 resistors potential difference between the terminals is V = IR = 1 x 8 = 8 V 14 Potentiometer Because resistance is directly proportional to the length of a resistor, a variable resistor also known as a potentiometer or as a “pot” can also be used to control the potential difference across some device. Sliding contact A can connect anywhere from one end to the other of the resistor chain. This way it can control voltage across a device and therefore the current through it, from maximum down to zero. 1. step is to do a circuit without device and then adjust point A in such a way that there is no current passing through potentiometer. Potential difference across potentiometer is 6 V. For some other battery point A would be somewhere else. If you include a lamp into circuit and the pointer is at A, potential difference across the lamp is zero. However, if the pointer is moved up to two-thirds the length of the potentiometer as in the figure, then the output voltage across the filament lamp would be ⅔ × 6V = 4V. Pots have a rotating wheel mounted in plastic and they are commonly used as volume and tone controls in sound systems. They can be made from wire, metal oxides or carbon compounds.