Supplementary Data
advertisement
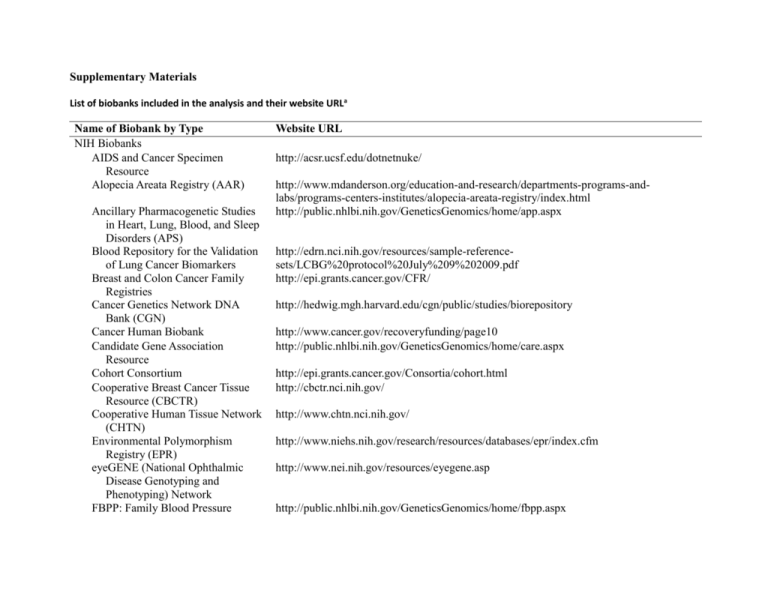
Supplementary Materials List of biobanks included in the analysis and their website URLa Name of Biobank by Type NIH Biobanks AIDS and Cancer Specimen Resource Alopecia Areata Registry (AAR) Ancillary Pharmacogenetic Studies in Heart, Lung, Blood, and Sleep Disorders (APS) Blood Repository for the Validation of Lung Cancer Biomarkers Breast and Colon Cancer Family Registries Cancer Genetics Network DNA Bank (CGN) Cancer Human Biobank Candidate Gene Association Resource Cohort Consortium Cooperative Breast Cancer Tissue Resource (CBCTR) Cooperative Human Tissue Network (CHTN) Environmental Polymorphism Registry (EPR) eyeGENE (National Ophthalmic Disease Genotyping and Phenotyping) Network FBPP: Family Blood Pressure Website URL http://acsr.ucsf.edu/dotnetnuke/ http://www.mdanderson.org/education-and-research/departments-programs-andlabs/programs-centers-institutes/alopecia-areata-registry/index.html http://public.nhlbi.nih.gov/GeneticsGenomics/home/app.aspx http://edrn.nci.nih.gov/resources/sample-referencesets/LCBG%20protocol%20July%209%202009.pdf http://epi.grants.cancer.gov/CFR/ http://hedwig.mgh.harvard.edu/cgn/public/studies/biorepository http://www.cancer.gov/recoveryfunding/page10 http://public.nhlbi.nih.gov/GeneticsGenomics/home/care.aspx http://epi.grants.cancer.gov/Consortia/cohort.html http://cbctr.nci.nih.gov/ http://www.chtn.nci.nih.gov/ http://www.niehs.nih.gov/research/resources/databases/epr/index.cfm http://www.nei.nih.gov/resources/eyegene.asp http://public.nhlbi.nih.gov/GeneticsGenomics/home/fbpp.aspx Name of Biobank by Type Program (FBPP) Framingham Heart Study Genetic Association Information Network Geneva (Gene Environment Association Studies) Liver (Hepatocellular Carcinoma) Reference Set Lupus Family Registry and Repository (LFRR) National Cell Repository for Alzheimer's Disease (NCRAD) National Database for Autism Research NHGRI Sample Repository for Human Genetic Research (NHGRI SRHGR) NIA Aging Cell Repository (NIA ACR) NIA Genetics of Alzheimer's Disease Data Storage Site NIDA Center for Genetic Studies (NIDA CGS) NIDCR Salivary Gland Tumor Biorepository NIDDK Central Repository NIGMS Human Genetic Cell Repository (NIGMS HGCR) NIMH Genetics Repository NINDS Human Genetics Repository PLCO Screening Trial Prostate Cancer Reference Set Website URL http://www.framinghamheartstudy.org/ http://www.genome.gov/19518664 http://www.genevastudy.org/ http://edrn.nci.nih.gov/resources/sample-reference-sets/edrn-hcc-reference-set.pdf lupus.omrf.org/ http://ncrad.iu.edu/ http://ndar.nih.gov/ndarpublicweb/ http://ccr.coriell.org/Sections/Collections/NHGRI/?SsId=11 http://ccr.coriell.org/Sections/Collections/NIA/?SsId=9 http://www.niageneticsdata.org/ http://zork.wustl.edu/nida/ https://www.fbo.gov/index?s=opportunity&mode=form&id=12767d6dfc166473ded6f15a e6d2d8eb&tab=core&_cview=1 https://www.niddkrepository.org/niddk/home.do http://www.nigms.nih.gov/Initiatives/HGCR/ http://www.nimhgenetics.org/ http://ccr.coriell.org/Sections/Collections/NINDS/?SsId=10 http://prevention.cancer.gov/programs-resources/groups/ed/programs/plco http://edrn.nci.nih.gov/resources/sample-reference-sets/Prostate%20Ref%20SOP.pdf Name of Biobank by Type Website URL Prostate SPORE National Biospecimen Network (NBN) Pilot Repository of Molecular Brain Neoplasia Data (REMBRANDT) Scleroderma Family Registry (SFR) SEER Residual Tissue Repository (RTR) The Breast Cancer Intergroup of North America The Database of Genotypes and Phenotypes (dbGaP) The Cancer Genome Atlas The eMERGE Network The Large-Scale Genome Sequencing Program The National Children's Study Women’s Health Initiative (WHI) SNP Health Association Resource Other U.S. Biobanks 23andMe Alpha-1 DNA and Tissue Bank Asterand http://prostatenbnpilot.nci.nih.gov/ http://caintegrator-info.nci.nih.gov/rembrandt http://www.uth.tmc.edu/scleroderma_reg/ http://seer.cancer.gov/biospecimen/ http://ctep.cancer.gov/investigatorResources/tbci/correlative_studies.htm http://www.ncbi.nlm.nih.gov/sites/entrez?db=gap http://cancergenome.nih.gov/ https://www.mc.vanderbilt.edu/victr/dcc/projects/acc/index.php/About http://www.genome.gov/10001691 http://www.nationalchildrensstudy.gov/Pages/default.aspx http://www.nhlbi.nih.gov/whi/ https://www.23andme.com/ http://www.alphaone.ufl.edu/dna_tissue_bank.php http://www.asterand.com/Asterand/human_tissues/frozenfixed.htm?gclid=CPem6cbbiJ4C FQjyDAodZQj6rA Autism Genetics Resource Exchange http://www.agre.org/ (AGRE) Bioserve Global Respository Human http://www.bioserve.com/globalRepository/globalRepositoryOverview.cfm Tissue Bank (and Serum Sample Bank) CCTS Virtual Biobank http://ccts.uth.tmc.edu/ccts-services/biobank CDC National Health and Nutrition http://www.cdc.gov/nchs/nhanes/genetics/genetic.htm Name of Biobank by Type Examination Survey Crohn's and Colitis Foundation DNA Data Bank Duke IGSP Biospecimen Repository El Camino Hospital MT Group Biobank HIHG Biorepository Hollings Cancer Center Tissue Biorepository Kaiser Permanente Research Program on Genes, Environment, and Health Mayo Clinic Biobank Medical College of Georgia Alzheimer's Disease DNA Bank Medical College of Wisconsin Pancreatic Cancer Tissue Bank Michigan Neonatal Biobank Mount Sinai Biobank National Psoriasis Victor Henschel BioBank Nephrology Research Biobank NUGene Origene Personalized Medicine Research Project PrecisionMed, Inc. SFARI Base South Carolina Cancer Center Tissue Bank The Accelerated Cure Project MS Website URL http://www.ccfadatabank.org/ http://www.genome.duke.edu/cores/biorepository/ http://www.elcaminohospital.org/Cancer_Center/Clinical_Trials/MT_Group_Biobank_Tis sue_Study/ http://www.mihg.org/weblog/core_resources/2007/11/mihg-biorepository.html http://hcc.musc.edu/research/sharedresources/biorepository/index.htm http://www.dor.kaiser.org/external/rpgeh/index.html http://mayoresearch.mayo.edu/mayo/research/biobank/ http://www.mcg.edu/alzres/questions.htm http://www.mcw.edu/AnnualReport2009/DiscoveryTissuebanktoadvanceclinicalcare.htm http://www.mnbb.org/ http://www.mountsinai.org/Research/Centers%20Laboratories%20and%20Programs/IPM/ IPM%20Research/Biobank http://www.psoriasis.org/NetCommunity/Page.aspx?pid=672 http://clinicaltrials.gov/ct2/show/NCT00381121 https://www.nugene.org/ http://www.origene.com/tissue/?gclid=CMSLjZrY5Z4CFQ_yDAodfG_oMw http://www.marshfieldclinic.org/chg/pages/default.aspx?page=chg_pers_med_res_prj http://www.precisionmed.com/about.html https://sfari.org/sfari-base http://www.palmettohealth.org/body.cfm?id=1010 http://www.acceleratedcure.org/repository/ Name of Biobank by Type Repository The Biorepository at The Feinstein Institute for Medical Research UC Davis Cancer Center Biorepository UConn Behavioral Gene Bank Non-U.S. Biobanks Biobank Japan CARTaGENE Chernobyl Tissue Bank Estonian Genome Project Genome Austria Tissue Bank Icelandic Biobank King's College London (KCL) Infectious Diseases BioBank Latvian Genome Project Medical Biobank Singapore Tissue Network UK Biobank Western Australian DNA Bank Website URL http://www.biorep.org/ http://ccresources.ucdmc.ucdavis.edu/csr/publicaccess.csr?pg=specimenhome http://gcrc.uchc.edu/Documents/RecruitmentAds/GCRC646%20Gene%20Bankbrochure. pdf http://www.src.riken.go.jp/english/index.html http://www.cartagene.qc.ca/index.php?lang=english http://www.chernobyltissuebank.com/ http://www.genomics.ee/index.php?lang=eng&PHPSESSID=ff45af7e1d8c3e94dd1be102 0517923b http://www.univie.ac.at/LSG/gatib/about.htm http://www.decode.com/ http://www.kcl.ac.uk/schools/medicine/research/diiid/centres/pii/biobank.html http://bmc.biomed.lu.lv/site/main/cgi/YaBB.cgi?action=lo http://www.biobanks.se/medicalbiobank.htmhttp://www.biobanks.se http://www.stn.org.sg/ http://www.ukbiobank.ac.uk/ http://www.wadb.org.au/ a The listed websites and links were active at the time of data collection; however, that may no longer be the case given the nature of online material. List of 65 Coding Questions Key Document Areas Coding Category Circle Response Y = Yes, specifically mentioned/allowed N = No, specifically not allowed NA = not addressed (If there is no definitive statement Y or N, but only discussion of the idea, then its NA) Q1. Document Type Select A or B and further define if B: A. Webpage A B. Document: B i. Actual consent form for research project i ii. Actual consent form for biobank specimen collection ii iii. Consent form template iii iv. Model consent form iv v. Guidebook/Manual v vi. Guidelines vi vii. Assent form template vii viii. Model assent form viii ix. Adverse Event document ix x. Script for Oral Consent x Q2. Study Population Guidelines xi. Policy document xi xii. Other xii; describe: May the following be included as participants? (A-F): A. Children/Adolescents (< 18) B. Adults (18+) (If document specifically states only those 18+ included, then A is “N”.) C. People whose competence is in question D. People with a prior medical condition E. People who are pregnant F. Controls G. In the signatures on the consent form is there a place for a parent, guardian, or some other type of witness to sign? Q3.Qualifications of the Biobank Researchers A. Does the document have instructions on clarifying the qualifications of the biobank researchers (i.e. listing credentials)? A. Y N NA B. Y N NA C. Y N NA D. Y N NA E. Y N NA F. Y N NA G. Y N NA A. Y N Q4. Clarification Statement B. Is medical attention available if necessary: B: 1. as part of the collection procedure? 1. Y N NA 2. due to an individual research result? 2. Y N NA 3. due to an incidental finding? 3. Y N NA A. Is there a clarification statement explicit on this document that clarifies the study is research? A. Y N B. Is the data from the project of clinical quality (e.g. – clinical data, data gathered in a clinic or by clinic staff)? B. Y N NA Q5. Research Results: A. Will screening individual research results be offered? A. Y N NA Specifies if disclosure of research results is addressed and if so, how and to whom B. Will study individual research results be offered? B. Y N NA; C. Definition/terms used to describe individual research results: C. list terms: D. Is there a time limit for return of IRRs? D. Y N NA E. Will aggregate research results be shared with participants? E. Y N NA F. May participants elect to know their IRRs? F. Y N NA G. May participants elect to NOT know their IRRs? G. Y N NA H. Will some IRRs be returned even if the participant refuses consent to receive or opts not to receive results? I. With whom will individual results be shared? 1. Individual Participants 2. Participant’s parent, guardian, or legal representative 3. Other family members 4. Primary Care Provider 5. Specialist Physician H. Y N NA I: 1. Y N NA 2. Y N NA 3. Y N NA 4. Y N NA 5. Y N NA; List specialist type: 6. Counselor/Psychologist/Mental health professional 7. Genetic Counselor 8. Other researchers Q6. Incidental Findings: Broad Statement (Specific to genetic research) A. Is there a broad statement about risks that may apply to incidental findings? B. Does anything about risks suggest that an incidental finding would receive further attention or would be disclosed? 6. Y N NA 7. Y N NA 8. Y N NA A. Y N B. Y N NA Q7. Incidental Findings: specifies if incidental findings are addressed and if so, how and to whom A. Is there a specific statement about identification of incidental findings? B. Definition/terms used to describe incidental findings C. Types of specific incidental findings addressed: A. Y N B: List definition/terms: C: List specifics: D. May participants elect to know if incidental findings were discovered? D. Y N NA E. May participants elect NOT to know if incidental findings were discovered? E. Y N NA F. Will some IFs be returned even if the participant refuses consent to receive or opts not to receive results? G. Is there a time limit for return of IFs? F. Y N NA H. Will the investigators consult with a specialist if an IF is found? If yes, specify:_____ I. Will IFs be disclosed to any of the following individuals? G. Y N NA H. Y N NA 1. Participant 2. Participant’s guardian 3. Primary care provider Specify: I: 1. Y N NA 4. Specialist physician 2. Y N NA 3. Y N NA 4. Y N NA 5. A counselor/ psychologist/mental health professional List type of specialist: 6. A genetic counselor 7. Other researchers 5. Y N NA 6. Y N NA 7. Y N NA Q8. Future Studies: specify if the document deals with future studies and if so how Researchers at the biobank: A1: Is participation in future biobank studies mentioned? A(1) Y N A2: Is the participant specifically asked if s/he elects to participate in future biobank studies? A(2) Y N NA Researchers outside the biobank: B1: Is participation in future studies by researchers at non-profit organizations outside the biobank mentioned? B(1) Y N B2: Is the participant specifically asked if s/he allows her/his data/samples to be used in future studies by non-profit researchers B(2) Y N NA outside the biobank? For-profit companies outside the biobank: C1: Is participation in future studies by researchers at for-profit organizations outside the biobank mentioned? C2: Is the participant specifically asked if s/he allows her/his data/samples to be used in future studies by for-profit researchers outside the biobank? C(1) Y N C(2) Y N NA D. Does the information explain the application and decision making process for future studies? E. Is the method of how the data/samples from the biobank will be shared with other researchers explained (e.g. – completely or partially de-identified)? D. Y N F. Is there a statement telling researchers how to cite the biobank in their research? E. Y N G. Future contact: 1) Is there an option for participants to choose to be contacted for future studies? 2) Is there a statement that the biobank may contact participants in the future (e.g. – to obtain updated medical info, a blood sample, or samples for additional studies?) F. Y N H1 Does the participant and/or his/her heirs receive compensation for discoveries made with his/her samples? H2 Does the researcher with whom the biobank shares the data/samples exercise intellectual property rights? G(1) Y N NA G(2) Y N H3 If yes, is there a time limit to the intellectual property rights? H4 May the researcher use the data/samples to develop commercial products? H1. Y N NA I. If the data or samples are shared with a researcher outside the biobank, can that researcher share them with a third party? J. Do participants have to contact the researcher to have their data/samples withdrawn from future research? K. Is it the case that samples/data already used cannot be withdrawn from the research? H2. Y N NA H3. Y N NA H4. Y N NA L. Does the biobank address what to do with an IRR discovered in a future study? M. Does the biobank address what to do with an IF discovered in a future study? N. Are researchers using biobank samples/data NOT allowed to attempt to identify the participant? O. Are researchers using biobank samples/data NOT allowed to test for certain IFs (e.g. non-paternity)? I. Y N NA J. Y N NA K. Y N NA L. Y N NA Describe: M. Y N NA Describe: N. Y N NA O. Y N NA; List: List: Q. 9 Other Interesting statements in the biobank documents related to IRR and IF (not covered in Q2-8)