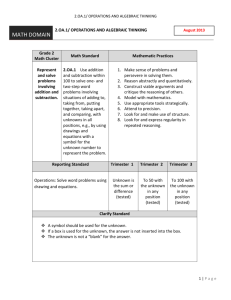
Chapter 1 Online Materials

Ecology 6e Chapter 9 Online Supplementary Materials
Applications
Estimating Abundance—From Whales to Sponges
The abundance of organisms and how abundance changes in time and space are among the most fundamental concerns of ecology. These factors are so basic that some authors define ecology as the study of distribution and abundance of organisms. Because abundance is so important, ecologists should understand how to estimate it for a wide variety of organisms. Keep in mind, however, that ecologists do not measure abundance as an end in itself but as a tool to understand the ecology of populations. Knowing the abundance of an organism can tell us whether its population is growing, declining, or stable. As we saw
(see text, chapter 9), population size is one of the characteristics that helps ecologists assess a species’ vulnerability to extinction. However, to estimate the abundance of species the ecologist must contend with a variety of practical challenges and conceptual subtleties. Some of these are discussed here.
Estimating Whale Population Size
In 1989, the journal Oceanus published a table that listed the estimated sizes of whale populations. The table included the following note: “All estimates . . . are highly speculative.” Why is it difficult to provide firm estimates of whale population size? Briefly, whales live at low population densities and may be distributed across vast expanses of ocean. They also spend much time submerged and move around a great deal. As large as they are, you cannot count all the whales in the ocean. Instead, marine ecologists rely on population estimation. Each method of estimation has its own limitations and uncertainties.
One method used to estimate population sizes of elusive animals involves marking or tagging some known number of individuals in the population, releasing the marked individuals so they will mix with the remainder of the population, and then sampling the population at some later time. The ratio of marked to unmarked individuals in the sample gives an estimate of population size. The simplest formula expressing this relationship is the Lincoln-Peterson index:
M/N = m/n where:
M = the number of individuals marked and released
N = the actual size of the study population m = the number of marked individuals in a sample of the population n = the total number of individuals in the sample
The major assumption of the Lincoln-Peterson index is that the ratio of marked to unmarked individuals in the population as a whole equals the ratio of marked to unmarked individuals in a sample of the population. If this is approximately so, then the population size is estimated as:
N = Mn/m
However, on average, the Lincoln-Peterson index overestimates population size. To reduce this tendency to overestimate, Bailey (1951,1952) proposed a corrected formula :
N = M (n + 1)/m + 1
Some of the assumptions of mark and recapture studies are:
•
•
•
•
All individuals in the population have an equal probability of being captured.
The population is not increased by births or immigration between marking and recapture.
Marked and unmarked individuals die and emigrate at the same rates.
No marks are lost.
Although real populations rarely meet all these assumptions, mark and recapture estimates of population size are often the best estimates available.
Whale populations have been studied using mark and recapture techniques for some time. In the early days of whale population studies, population biologists marked whales by shooting a numbered metal dart into their blubber. The idea was that the dart would be recovered when marked whales were caught and processed during whaling operations. However, the accuracy of this method was limited by several factors.
First, it is difficult to mark a free-swimming whale from a moving boat on the open sea, and so biologists never knew exactly how many whales shot at were actually marked. Second, the recovery of darts during processing was poor. It is easy to overlook a relatively small dart on a huge whale. Experiments showed that Japanese whalers recovered 60% to 70% of marks from whales known to be marked, while other whaling fleets recovered an even lower proportion. Still, the greatest limitation of this early mark and recapture technique is that it requires killing whales during the recapture phase, which is unacceptable when studying protected or endangered species.
Refined mark and recapture methods do not require artificially marking or capturing whales. In the
“marking” phase of newer procedures, a whale is photographed and its distinguishing marks are identified.
These photographs, along with information such as where the photograph was taken and whether the whale was accompanied by an offspring, are catalogued for future reference. In the “recapture” phase the whale is photographed at a later date and identified from previous photos. This method is called photoidentification.
For more than three decades, Steven Katona and his colleagues (Katona 1989, Stevick et al. 2003) have used photoidentification to study the humpback whales, Megaptera novaeangliae, of the North
Atlantic (fig. 1). Humpback whales are particularly rich in individual marks, especially on the tail or flukes.
This is convenient for photographic studies because humpback whales generally raise their flukes above the water before they dive. This behavior, called “fluking,” exposes the flukes to the photographer and reveals potentially unique markings (fig. 2).
Figure 1 A humpback whale, Megaptera novaeangliae . © Getty RF.
(a)
(b)
Figure 2 Unique markings on the flukes of humpback whales (a) and (b) identify them as different individuals. a-b © Getty RF.
Katona points out that the unique markings on a humpback whale’s flukes result from a combination of genetics and accidents. In humpback whales, fluke pigmentation ranges from completely black to white.
This variation in pigmentation probably reflects genetic differences between individuals. Injuries often produce scars that superimpose other marks on the basic pigmentation of the flukes. Injuries may be the result of fighting between humpback whales, attacks by sharks, or attachment by parasites. The scars produced when these injuries heal create black marks on white flukes and white marks on black flukes.
Using photographs of these marks, Katona and his colleagues began compiling the North Atlantic
Humpback Catalog, which grew, by 2008, to include the photographs of more than 6,000 individual humpback whales. The photographs included in the catalog, along with information on where each photograph was taken, whether the whale was accompanied by an offspring, and other available observations, are curated for future reference. This photographic record is an invaluable source of
information for determining the migration routes, feeding grounds, breeding grounds, and size of the North
Atlantic humpback whale population (fig. 3).
Figure 3 Photoidentification and the North Atlantic humpback whale population.
From 1979 to 1986, Scott Baker, Janice Straley, and Anjanette Perry (1992) photographed and identified 257 humpback whales along the coast of southeastern Alaska. In one part of their study the researchers used photoidentification to estimate the number of humpback whales in Frederick Sound,
Alaska. In their first sampling period, from July 31 to August 3, 1986, the team photographed and identified 72 humpback whales. In a second sampling period, from August 29 to September 1, 1986, they photographed and identified 78 humpback whales. Of the 78 whales photographed in the second sampling period, 56 were photographed for the first time, while 22 had also been photographed during the first sampling period. These 22 whales were the “recaptures.” We can use these data and the corrected Lincoln-
Peterson index to estimate the total number of whales in Frederick Sound from August 29 to September 1,
1986:
N = M (n + 1)/m + 1 = 72 (78 + 1)/22 + 1 = 247
In other words, Baker and his colleagues estimated that there were 247 humpback whales in Frederick
Sound during the time of their study. Population estimates such as this are very important for monitoring the state of populations. In addition, photographic studies provide information on movements, calving intervals, and survival because photoidentified whales can go on yielding information throughout their lives.
Population ecologists have now applied the photoidentification techniques to several whale species including gray, right, blue, fin, sei, and killer whales. For instance, by 1986, scientists had photographed about 200 of the 300 to 400 North Atlantic right whales remaining in the Atlantic. From the growing photographic record of the North Atlantic right whale population, ecologists have estimated a 4- to 7-year calving interval for the population. This long period between offspring alone gives a clue to why this population is so vulnerable to extinction in the face of whaling pressure. In another study, population ecologists photographed and identified the entire population of 325 killer whales in Puget Sound and around Vancouver Island, revealing information about births, deaths, and the size of the population. An advance in this research approach is the use of computerized image analysis to help identify and match photos of whales. While not providing all the information necessary for conserving and managing whale populations, photographic studies are clearly making an important contribution.
Though it may be more challenging physically, the process of counting whales is much like counting many other kinds of animals such as humans, lynxes, trout, or lady beetles. However, ecologists must use different methods to estimate the abundance of organisms that have a more variable growth form or differ greatly in size. As we shall see in the next example, this is particularly true when the relative abundances of very different organisms are compared.
The Relative Abundance of Corals, Algae, and Sponges
The reefs along the north coast of Jamaica were once dominated by corals. Thickets of staghorn coral rose from the seafloor like marine bramble bushes, and elkhorn coral grew in abundance in the surging waves.
Then dramatic change came in 1980 with Hurricane Allen, which generated waves large enough to flatten the staghorn coral thickets and dislodge elkhorn corals. Most of the branching corals in shallow water were reduced to rubble. However, Hurricane Allen and its devastation was not the only problem. The reefs seemed to be changing even at depths below 25 m, where there was little hurricane damage. Assessing the extent and nature of changes on the reef would require detailed population studies.
Fortunately, Terence Hughes (1996) had started long-term studies of coral populations near Discovery
Bay, Jamaica, in 1977, 3 years before Hurricane Allen. One of his goals was to document and understand apparent shifts in dominance from corals to algae. He was also concerned with documenting possible changes in sponge populations. But how can the relative abundance of organisms so different in size and growth form be estimated? A coral colony may cover several square meters or just a few square centimeters. Sponges also differ greatly in size. Algae of several species may grow together in a tangled mat covering several square meters or as a few isolated individuals. Ecologists studying terrestrial plants encounter similar size differences among plants of different ages and species. In the face of this variation, ecologists resort to measures of abundance that take into account differences in size. For instance, ecologists studying shrubs, herbaceous vegetation, or marine organisms such as corals, algae, and sponges often measure coverage, the area of landscape or reef covered by a species.
Hughes estimated percent cover by corals, algae, and sponges on his study reef nearly every year from
1977 to 1993. He made his estimates from photographs of 12 study plots 1 m2 in size taken from a standard distance using slide film. He then mapped the positions and sizes of all coral and sponge colonies within the quadrats. He used a computer program to measure the areas on his maps that were covered by corals and sponges and then converted these measurements of absolute area to percent cover. Hughes estimated percent cover of algae by projecting the slide images of his study plots on a screen and superimposing a uniform grid of points on the image. The grid contained 100 points per square meter. The percentage of these points contacting algae in the image gave him an estimate of percent algal cover.
Hughes’ 16-year study shows clear changes in the abundances of corals and algae (fig. 4). During the study, algal cover increased tenfold from 7% to 76%. At the same time, coral cover decreased from about
48% to 13%, while sponge populations remained fairly constant. Verifying the status of populations such as
these is one of the most basic aspects of ecological research. Such measurements are the first step toward identifying the factors that determine the distribution and abundance of organisms. With his photographs
Hughes was able to show that this shift from a coral-dominated reef to one dominated by algae was due to increased mortality of coral colonies and reduced recruitment of new corals to the population. These are aspects of population dynamics that we cover in chapter 10.
Figure 4 Estimating abundance as percent cover: corals, sponges, and algae (data from Hughes 1996).
References
Bailey, N. T. J. 1951. On estimating the size of moblile populations from recapture data. Biometricka
38:293-306.
Bailey, N T. J. 1952. Improvements in the interpretation of recapture data. Journal of Animal Ecology
21:120-27
Hughes, T. P. 1996. Demographic approaches to community dynamics: a coral reef example. Ecology
77:2256-60.
Katona, S. Getting to know you. Oceanus 32:37-44.
North Atlantic Humpback Whale Catalog. http://www.coa.edu/html/hahc.htm
Stevick, P. T., J. Allen, P. J. Clapham, N. Friday, S. K. Katona, F. Larsen, J. Lien, D. K. Mattila, P. J.
Palsboll, J. Sigurjonsson, T. D. Smith, N. Oien, and P. S. Hammond. 2003. North Atlantic humpback whale abundance and rate of increase four decades after protection from whaling. Marine Ecology
Progress Series 258:263-73.