Bright Lights Lab
advertisement

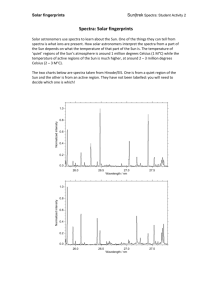
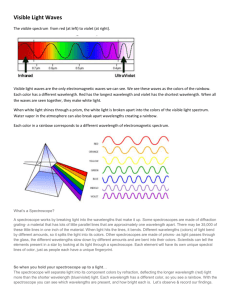
Bright Line Spectra of Various Elements Caution: In this lab, you will be working with a highly flammable liquid, methanol. Care must be taken when doing this lab. Safety glasses and aprons must be worn at all times. Misbehavior will not be tolerated. Pre-Lab: Read the lab procedure and introduction, and set-up your lab notebook with the following information… Title Partner Objective Data Tables Introduction: When an element is energized, either by high temperature or electromagnetic radiation, it will emit light of definite wavelengths. This light can be separated into its component wavelengths by the use of a spectroscope. The spectroscope will show each wavelength of light as a bright line. This arrangement of bright lines is called bright line spectra and is characteristic for each element. The wavelength of a light can be measured in several different units. You may see it expressed in nanometers (nm), meters (m), or Angstroms (Å). These are some important conversion factors: 1.0 nanometer = 1.0x10-9 meters 1.0 Angstrom = 1.0x10-10 meters 1.0 Angstrom = 0.10 nanometers The spectroscope we will be using for this lab measures the wavelength of the bright line spectra in thousands of angstroms. Pre-Lab: You must be familiar with how to use a spectroscope in this lab activity. Your teacher will demonstrate how to use it. Once you have used the spectroscope correctly with your instructor on the demonstrated gas tube, you should proceed to record the spectra of the other samples in gas tubes around the side of the room. Record these spectra on a piece of graph paper. Use colored pencils to provide an accurate description of the spectra. Label the wavelength of the light you observe for each colored line. Materials: Methanol Lithium chloride Calcium chloride matches Sodium chloride copper chloride spectroscopes Potassium chloride unknown gas lamps safety glasses lab apron Procedure: Both lab groups at the lab table will use the same tray and will test each compound simultaneously. Test ONLY ONE compound at a time. Part A: 1. In the tray at your lab table are labeled dishes with a sample of a compound of the element to be studied. 2. To the first dish, add a small amount of methanol (just enough to wet the substance). Do not over saturate with methanol or results will be stinky-pants. 3. Light a match and place near the methanol in the dish. As soon as the methanol begins to burn, remove the match (and your hand). Put the match out but be careful not to blow towards burning sample. CAUTION: Do not add methanol while the compound is actively burning!!! 4. As you watch the methanol burn, prepare to use the spectroscope. As the compound becomes heated, the flame will begin to change colors and will emit light of a characteristic color for the element being tested. 5. One lab partner should record data, while the other lab partner uses the spectrometer to measure the color and wavelength of the light emitted from the element. The line spectra may be faint and flickering. Do your best to get an accurate wavelength measurement for each line. Record both the color and wavelength for each bright line in your data table. 6. If there is more than one colored line, record the color and wavelength measurement for every color line you see. 7. Record both the color and the wavelength for each bright line in your data table. 8. It may be necessary to repeat the procedure in order to get an accurate reading. Repeat the procedure only after all of the methanol has burned off and the flame is out. 9. Repeat steps 1-8 for each given sample of the compound. Part B: 1. There is a gas tube containing an unknown located on the front demo table. Observe this unknown with your spectroscope. 2. Measure and record the color and wavelengths of the light emitted from this element in your data table. 3. After you have measured the unknown, use the bright line spectra chart in the laboratory to determine what element was in the gas tube. Part C: Clean Up!!!! 1. Leave the compounds in the dishes. They can be used for the next lab group. 2. Wash off your table so that no methanol or accidental spills from chemicals remain. 3. Throw out all used matches and return remaining matches to your instructor. Data Table: compound Lithium Chloride Sodium Chloride Potassium Chloride Calcium Chloride Strontium Chloride Copper Chloride Unknown Gas color of flame diagram of bright line spectrurm color of lines wavelengths of lines Background Information: How is a continuous spectrum different from an atomic emission spectrum? Which are you observing in this lab experiment? What kind of information does the atomic emission spectrum give us about an atom of a specific element? Calculations: Your measurements in lab were given in thousands of Angstroms (if you have a 3.2 written down, then that means it was 3200 Å) Report the wavelength for your two favorite spectra in meters using the factor label method and the conversion factors you found in the introduction to this lab. Questions: 1. Based on your measurement of the bright line spectra and using available reference materials, what is the identity of your unknown? Why did you decide on this element? 2. Which elements contained high energy electrons? Which elements contained low energy electrons? How could you tell? 3. Briefly explain why, when an element is heated, it gives off line spectra of certain wavelengths. 4. How are bright line spectra useful? (Hint: Unknowns! Astronomy!!) 5. State and explain any sources of error.