90173 Describe the chemistry of selected non-metals and
advertisement
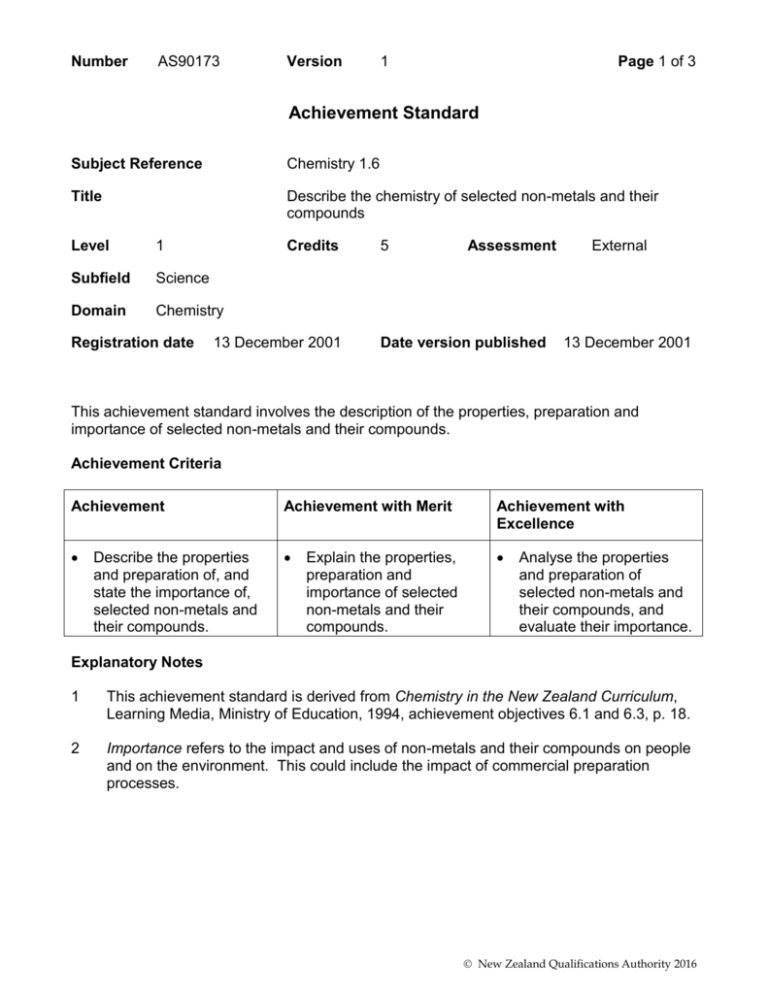
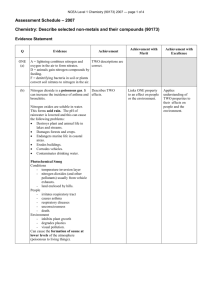
Number AS90173 Version 1 Page 1 of 3 Achievement Standard Subject Reference Chemistry 1.6 Title Describe the chemistry of selected non-metals and their compounds Level 1 Subfield Science Domain Chemistry Registration date Credits 13 December 2001 5 Assessment Date version published External 13 December 2001 This achievement standard involves the description of the properties, preparation and importance of selected non-metals and their compounds. Achievement Criteria Achievement Achievement with Merit Achievement with Excellence Describe the properties and preparation of, and state the importance of, selected non-metals and their compounds. Explain the properties, preparation and importance of selected non-metals and their compounds. Analyse the properties and preparation of selected non-metals and their compounds, and evaluate their importance. Explanatory Notes 1 This achievement standard is derived from Chemistry in the New Zealand Curriculum, Learning Media, Ministry of Education, 1994, achievement objectives 6.1 and 6.3, p. 18. 2 Importance refers to the impact and uses of non-metals and their compounds on people and on the environment. This could include the impact of commercial preparation processes. New Zealand Qualifications Authority 2016 Number AS90173 Version 1 Page 2 of 3 3 Non-metals will involve a selection from the following: properties of carbon, chlorine, nitrogen and sulfur - state at room temperature, colour, solubility in water and reaction with oxygen processes of commercial preparations: chlorine (electrolysis of brine); nitrogen (from liquid air); sulfur (from natural gas) carbon and nitrogen cycle - the description of the nitrogen cycle will be limited to the major nitrogen-containing species (nitrogen gas, ammonia, nitrate, nitrite and proteins) and the changes involved allotropes of carbon and sulfur, for carbon the relationship of their structure to their physical properties uses of chlorine related to the nature of its aqueous solution. 4 Non-metal compounds will involve a selection from the following: properties of the compounds - carbon dioxide, sulfur dioxide, nitrogen dioxide, nitric acid and sulfuric acid: solubility and the acidic nature of their aqueous solution laboratory preparations: ammonia (by heating a mixture of calcium hydroxide and ammonium chloride); hydrogen chloride (from the reaction of sodium chloride and concentrated sulfuric acid); sulfur dioxide (by the reaction of dilute hydrochloric acid with sulfites); oxides of nitrogen (from the reaction of copper with nitric acid) processes of commercial preparations: ammonia (by the Haber Process); sulfuric acid (by the Contact Process); superphosphate (from rock phosphate); sodium hypochlorite (from sodium hydroxide and chlorine) uses of non-metal compounds related to their properties. The compounds will be selected from carbon dioxide, sulfur dioxide, sodium hypochlorite. The uses are related to: CO2 - solubility in water, density and inability to support combustion; SO2 - solubility in water, and its ability to act as a reductant (in its capacity as a preservative and bleach); sodium hypochlorite - its bleaching and antiseptic properties the impact of non-metal oxides on people and the environment. The oxides will be selected from carbon dioxide, nitrogen dioxide and sulfur dioxide. Impact on people and the environment is limited to the effects of global warming, photochemical smog and acid rain. 5 For achievement with excellence, the discussion will include the relationship between the properties of non-metals and their compounds, and their importance. 6 Balanced equations for reactions may be required, where appropriate. New Zealand Qualifications Authority 2016 Number AS90173 Version 1 Page 3 of 3 Quality Assurance 1 Providers and Industry Training Organisations must be accredited by the Qualifications Authority before they can register credits from assessment against achievement standards. 2 Accredited providers and Industry Training Organisations assessing against achievement standards must engage with the moderation system that applies to those achievement standards. Accreditation and Moderation Action Plan (AMAP) reference 0226 New Zealand Qualifications Authority 2016