Update on Azole Antifungals
advertisement

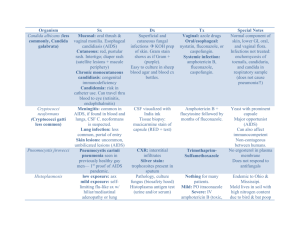
Update on Azole Antifungals Dimitrios I. Zonios; John E. Bennett Semin Respir Crit Care Med. 2008;29(2):198-210. ©2008 Thieme Medical Publishers Posted 06/11/2008 Abstract and Introduction Abstract This is a comprehensive, clinically oriented review of the four commercially available triazoles: fluconazole, itraconazole, voriconazole, and posaconazole. Emphasis is placed in pharmacology, drug interactions, adverse events, antifungal activity, and the evolving perspective of their clinical use. Key clinical trials are briefly discussed, and specific drug indications summarized. Fluconazole remains a valuable low-cost choice for the treatment of various fungal infections, including candidiasis and cryptococcosis. It has relatively few drug interactions and is safe but lacks activity against filamentous fungi. The use of itraconazole is historically plagued by erratic bioavailability of the oral capsule, improved with the oral solution. Drug interactions are numerous. Itraconazole exhibits significant activity against Aspergillus and the endemic fungi. Voriconazole has revolutionized the treatment of aspergillosis in severely immunocompromised patients, but its use is compromised by complicated pharmacokinetics, notable drug interactions, and relatively significant adverse events. Finally, posaconazole is the last addition to the azole armamentarium with extended antifungal spectrum, significant activity against the zygomycetes, and, apparently, optimal safety profile. Posaconazole has a significant role for the prophylaxis of invasive fungal infections in severely immunocompromised patients. Multiple daily dosing, a need for fatty foods for absorption, and absence of an intravenous formulation restrict its use to selected populations. Introduction The azole antifungals include two classes, imidazoles and triazoles, which share the same mechanism of action. Imidazoles have a two-nitrogen azole ring; they are predominantly used topically and have been replaced for systemic administration by triazoles, which have three nitrogens in the azole ring. Triazoles have a more favorable pharmacokinetic profile than imidazoles and do not significantly inhibit human sterol synthesis. They were introduced almost 30 years ago. Triazoles include fluconazole, itraconazole, voriconazole, and posaconazole (Figure 1), each of which will be discussed in detail. Fluconazole and itraconazole were the first azoles in clinical practice. Newer azoles such as voriconazole and posaconazole have been developed to overcome the limited efficacy of fluconazole against Aspergillus and other molds and to improve absorption, tolerability, and drug interaction profile of itraconazole. Voriconazole is structurally similar to fluconazole and posaconazole to itraconazole. Figure 1. Commercially available triazoles. Mechanism Of Action Triazoles inhibit the cytochrome P450 enzyme 14-α-sterol-demethylase. This enzyme is implicated in the biosynthetic pathway of ergosterol, which is an essential molecule of the fungal cell membrane. Inhibition of this enzyme leads to accumulation of 14-amethylsterols on the fungal surface, which results in arrest of fungal growth. Triazoles are generally considered fungistatic. Table 1 provides formulations of the commercially available triazoles. Pharmacology Fluconazole Fluconazole is water soluble and it is very well absorbed from the gastrointestinal tract.[1] Its absorption is not affected by food or gastric pH. In blood, 12% is protein bound. Eighty percent is excreted unchanged in the urine; hepatic CYP2C9 enzyme plays a minor role in its metabolism. Fluconazole diffuses readily in body fluids and cerebrospinal fluid (CSF) concentration is ∼70% of serum. Terminal plasma elimination half-life of fluconazole is ∼30 hours after oral administration.[1] The dose should be halved for creatinine clearance below 50 mL/min, and 100% of the regular dose should be given after hemodialysis. An initial double dose is recommended as a loading dose but is not always used. Itraconazole Itraconazole is water insoluble, and its bioavailability is variable after oral ingestion. The capsule is best absorbed with food. The oral solution is better absorbed in the fasting state[2] and generally achieves higher bioavailability than the capsule.[3] Unpredictable bioavailability of the oral solution is seen in bone marrow transplants and human immunodeficiency virus (HIV) patients.[4] Itraconazole is solubilized in the oral solution and the intravenous form by a hydroxypropyl-β-cyclodextrin. This pharmacologically inactive oligosaccharide molecule acts as a vehicle for the hydrophobic drug, which is readily released upon contact to the lipid membrane of the enteric cells. After absorption, itraconazole is found in equal amounts in plasma as native drug and the hydroxy-itraconazole metabolite. Unlike metabolites of other azoles, hydroxyitraconazole has equivalent antifungal activity to the parent compound. Both molecules are bound more than 99% to plasma proteins. Itraconazole is metabolized in the liver and is a substrate and strong inhibitor of CYP3A4. Elimination half-time is ∼30 to 40 hours after both oral and intravenous administration, and steady-state concentration is reached after 4 days of treatment. Itraconazole does not penetrate the CSF. Due to the potential accumulation of the hydroxypropyl-βcyclodextrin intravenous vehicle of itraconazole, patients with creatinine clearance of less than 30 mL/min should not be treated with intravenous itraconazole. Voriconazole Voriconazole is poorly soluble, despite structural similarity to fluconazole. Following oral administration, as either tablet or solution, bioavailability is 96%. Oral absorption is reduced by 22% when taken with food. Solubility of the intravenous formulation is achieved by sulfobutyl ether β-cyclodextrin (SBECD), a molecule pharmacologically similar to hydroxypropyl β-cyclodextrin used for the itraconazole intravenous formulation. The oral solution does not contain SBECD. Voriconazole is 56% bound to serum proteins. The volume of distribution is good (4.6 L/Kg), and the drug is metabolized in the liver, mainly by CYP2C19 and CYP3A4 and to a lesser extent by CYP2C9.[5] Steady-state concentration without a loading dose is achieved within 5 to 6 days of treatment. Voriconazole penetrates the blood-brain barrier, and CSF level is ∼46% of the serum level. The percentages of voriconazole level in noninflamed vitreous and aqueous humor are 38.1% and 53.0%, respectively.[6] The major metabolic pathways in humans involve fluoropyrimidine N-oxidation, fluoropyrimidine hydroxylation, and methyl hydroxylation. The main metabolite in serum is the inactive N-oxide.[7] Only 2% of the active drug can be found in urine. Voriconazole exhibits nonlinear pharmacokinetics due to saturation of metabolism, and small increases in dose result in exponential increases in blood levels. Optimal therapeutic levels of voriconazole have not been described, but in phase II/III studies a median trough voriconazole level of 2.49 µg/mL was found. Although high voriconazole blood levels have been implicated in increased toxicity[8-10] and low levels to disease progression,[11] therapeutic and toxic levels of this agent are yet to be determined. Moreover, correlation between dose and levels is weak, and there is significant inter- and intrapatient voriconazole level variability.[12] Voriconazole pharmacokinetics are also affected by, at least, CYP2C19 enzyme genetic variability.[13] Nineteen percent of the Asian and 2% of the Caucasian population are poor metabolizers in terms of CYP2C19 activity[14] (homozygous for the CYP2C19 allele) resulting in high voriconazole blood levels. Higher percentages of patients are heterozygous for the CYP2C19 allele, leading to an intermediate metabolizer phenotype with moderately increased voriconazole blood levels. Patients with mild to moderate hepatic disease (Child-Pugh class A and B) should get 50% of the usual maintenance dose. The safety of voriconazole has not been established for liver cirrhosis and should not be used in this setting. Finally, due to accumulation of the intravenous voriconazole vehicle SBECD, the drug should be used with caution in patients with creatinine clearance of < 50 mL/min. Posaconazole Posaconazole is available only as a suspension for oral use. Absorption is increased when taken with food, especially fatty meals,[15] and is not affected by use of antacids. In detail, the area under the curve (AUC) and maximum concentration (Cmax) was increased fourfold with a fatty meal and between 2.6 and 2.9 times with a nonfatty meal, as compared with the fasting state.[15] Moreover, oral absorption is better in divided daily doses and optimal when administered four times daily.[16] Absorption is saturated for doses more than 800 mg daily and is diminished in patients undergoing allogeneic bone marrow transplantation.[17] Posaconazole is more than 98% protein bound, and its volume of distribution is between 343 and 1341 L, compatible with good tissue penetration. The mean half-life of posaconazole is more than 20 hours, and the drug is metabolized in the liver through glucuronidation (phase 2 metabolism) and excreted primarily in bile (76.9%) and in urine (14%) as unchanged drug or inactive metabolites. Although posaconazole is not metabolized through liver CYP450 system enzymes (phase 1 metabolism), it is a moderate inhibitor of CYP3A4,[18] which leads to increased blood levels of drugs using the CYP3A4 metabolic pathway when coadministered. There is no need for dose adjustment in patients with moderate to severe renal disease, and the drug is not cleared with hemodialysis. Posaconazole has not been adequately studied in patients with hepatic insufficiency and should be used with caution in this setting. Drug Interactions Triazoles have numerous clinically significant drug interactions, the list is constantly expanding and the majority of those currently identified are presented in Table 2 [19-72] and Table 3 .[47,48,50-53,56,73-80] Most of these interactions arise from competitive inhibition of liver oxidative metabolism via rapid reversible binding to CYP450 system enzymes (phase I metabolism). Triazoles interfere mainly with CYP3A4, CYP2C9, and CYP2C19 with distinctive affinity ( Table 4 ) and, accordingly, they exhibit slightly different drug interaction profiles. In addition, itraconazole and posaconazole, but not fluconazole and voriconazole, are inhibitors of gastric P-glycoprotein.[81] P-glycoprotein is a transmembrane efflux pump limiting systemic exposure to many drugs by inhibiting GI absorption. Thus inhibition of P-glycoprotein by azoles may lead to increased systemic exposure of drugs affected by this transport system. Because interactions caused by itraconazole have been extensively studied and both itraconazole and posaconazole inhibit CYP3A4, drugs with known interaction to itraconazole should be used with caution in posaconazole-treated patients. Among the most significant common drug interactions of triazoles are drug elevations of cyclosporine, tacrolimus, and sirolimus, most Ca channel blockers, most benzodiazepines, many statins and steroids, warfarin, and rifabutin ( Table 2 ). Carbamazepine, phenobarbital, phenytoin, rifampin, and rifabutin significantly decrease azole concentrations ( Table 3 ). Increased blood levels of terfenadine, astemizole, cisapride, pimozide, and quinine can cause Q-Tc prolongation and predispose to torsades de pointes. Finally, increased cytotoxic chemotherapy-related toxicity can be caused by concomitant treatment with triazoles (with the exception of fluconazole) and vinca alkaloids, cyclophosphamide, vinorelbine, and busulfan. In general, use of an azole should be cautious if an interaction is suspected. The absence of data to support an interaction does not mean absence of interaction, especially with the newer azoles. Adverse Events Fluconazole Fluconazole is generally well tolerated. Serious adverse events, mainly liver toxicity, are rare. Headache, nausea, vomiting, abdominal pain, and diarrhea are among the most common complaints. Skin rashes can also occur. In patients with prolonged treatment, headache, alopecia, and anorexia are common as well as eosinophilia and aspartate aminotransferase (AST) elevation.[82] Alopecia is reversible and dose dependent, commonly seen in chronic treatment with doses above 400 mg daily. [83] Liver toxicity can rarely lead to acute hepatic necrosis. Rare adverse events, typically restricted to a few case reports, are agranulocytosis, thrombocytopenia, toxic epidermal necrolysis, Stevens-Johnson syndrome, anaphylaxis, Q-T prolongation and torsades de pointes. Fluconazole is a category C drug; its use should be avoided in pregnancy due to teratogenicity in animals and a few reports of severe organ deformities in humans.[84] Itraconazole Itraconazole most often causes nausea and vomiting, and these symptoms are dose dependent. Dividing the dose increases tolerance. Other common adverse events include AST/ALT (alanine aminotransferase) elevation, hypokalemia, and rash.[85] Hypokalemia can be severe, especially in high doses (> 400 mg daily) and accompanied by hypertension and dependent edema.[86] The oral solution is generally less well tolerated due to the presence of cyclodextrin, which can cause diarrhea and gastrointestinal discomfort, possibly due to osmotic effect. Serious hepatotoxicity can lead to liver failure but is rare. Heart failure is caused by a direct negative inotropic effect and has led to a "black box" warning on the label.[87] Intravenous itraconazole can cause chemical phlebitis. Use in pregnancy and lactation is contraindicated (class C). Voriconazole Voriconazole is usually well tolerated; the most common adverse events after voriconazole treatment are visual changes, with an incidence of 20 to 23%.[88,89] Occurrence of 44.8% has been reported in one study.[90] Typically, the patients report blurred vision, photopsias, photophobia, and color changes 30 minutes to 1 hour after dosing. The symptoms are transient and reversible, almost never lead to drug discontinuation, and tend to dissipate after repeated dosing. As with all azoles, voriconazole can cause hepatotoxicity, mainly manifested by elevation of AST/ALT, which, on rare occasion, can be serious or lethal.[91] Both visual changes and hepatotoxicity have been associated with high voriconazole blood levels.[9] Visual and, rarely, auditory hallucinations can occur in less than 5% of patients[88] and should be distinguished from visual changes. The patient is usually alert and has awareness of the symptoms, which is rarely described as something unpleasant. Hallucinations are most often reported with the intravenous formulation and tend to disappear with the oral drug or with continued treatment. Confusion is less common. Skin rashes and cheilitis can occur after prolonged treatment, mainly due to photosensitivity. Although most commonly the rash is restricted to facial erythema in sun-exposed areas, more serious eruptions can occur necessitating discontinuation of the drug.[92] Pseudoporphyria, toxic epidermal necrolysis, and multifocal facial squamous cell carcinomas have also been reported.[93-95] Use of sunscreen should be advised, and appropriate guidance for sun exposure avoidance should be given in chronically treated patients. Q-Tc prolongation and torsades de pointes should be a consideration in patients with predisposing factors.[96] Finally, acutely painful extremities and peripheral neuropathy have been described.[97] Voriconazole is teratogenic in animals and should not be used during pregnancy (class D). Posaconazole The safety profile of posaconazole seems to be more favorable than that of voriconazole. Common adverse events are headaches and gastrointestinal complaints, such as nausea, diarrhea, and vomiting (3 to 12% each in several studies).[98-102] Hepatotoxicity and liver enzyme abnormalities have been reported in less than 2 to 3% of patients,[101,102] hypokalemia in 3%, and rash in 3%.[99] Q-Tc prolongation was reported to be as high as 4% in one study.[99] Long-term posaconazole treatment seems to be equally safe, with no increase or additional adverse events observed. [103] The safety of posaconazole has not been evaluated in children under 13 years old. Because skeletal malformations have been reported in posaconazole-treated rats, use in pregnancy should be avoided (class C drug). Antifungal Activity-Therapeutic Uses Approved indications in the United States for the commercially available azoles and respective doses are presented in Table 5 . An estimation of the relative daily oral cost of triazole usage is given in Table 6 . Fluconazole Fluconazole is active against Candida spp. (except Candida glabrata and krusei), Cryptococcus neoformans, Blastomyces dermatitidis, Coccidioides spp., Histoplasma capsulatum, Sporothrix schenckii, and ringworm. It is first-choice treatment for certain Candida and cryptococcal infections as well as nonmeningeal coccidioidal infections. Fluconazole as a single dose of 150 mg is effective for the treatment of vaginal candidiasis. Oropharyngeal and esophageal candidiasis can be treated with 100 to 200 mg daily for 14 to 21 days. A loading dose of 200 to 400 mg can be given the first day. Fluconazole has an indication and has been shown to be as effective as amphotericin B for the treatment of candidemia in nonneutropenic patients.[104] One post hoc analysis indicated that more severely ill patients with candidemia benefited by starting with the addition of amphotericin B to fluconazole.[105] Guidelines from the Swiss Consortium do not recommend fluconazole for patients with septic shock due to candidemia.[106] The emergence of fluconazole-resistant Candida species during prior fluconazole exposure and the introduction of echinocandins in clinical practice make fluconazole a less attractive choice. In two studies with patients undergoing bone marrow transplantation, prophylactic use of fluconazole has been shown to reduce the incidence of systemic and superficial fungal infections.[107,108] In one of these studies, fluconazole prophylaxis reduced the need for amphotericin B empirical treatment, and survival was increased. In these studies, a dose of 400 mg daily has been used, although one study has concluded that 200 mg may be equally effective.[109] One study has concluded that fluconazole prophylaxis decreases fungal colonization and invasive fungal infection in preterm infants.[110] In a recent study, a dose of 6 mg or 3 mg fluconazole per kg reduced the incidence of colonization and invasive Candida infections in neonates weighing less than 1500 g at birth compared with placebo. [111] Overall mortality was the same. Fluconazole is also used for the treatment of cryptococcal meningitis in HIV- and nonHIV-infected patients. In HIV infection, 400 mg of fluconazole daily is given for 8 weeks following a 2 week amphotericin B ± flucytosine combination therapy. Fluconazole is then continued indefinitely at 200 mg daily, or as long as the CD4 count remains below 100/mm3.[112] In the non-HIV-infected and in alert and well-oriented HIV patients fluconazole can be used as initial treatment.[113] Nonmeningeal cryptococcosis can be treated with fluconazole in both HIV and non-HIV patients at a dose of 400 mg daily for 6 to 12 months. Treatment of coccidioidomycosis with fluconazole is indicated for the meningeal form due to adequate CSF penetration and limited toxicity compared with amphotericin B. Therapy is lifelong at doses of at least 400 mg daily. Although fluconazole is also effective for nonmeningeal forms of this disease, itraconazole is preferred due to possible increased efficacy for skeletal infections.[114] Itraconazole Itraconazole is active in vitro and in vivo for Aspergillus fumigatus, Candida spp., Cryptococcus neoformans, Trichophyton spp., and the dimorphic fungi (Blastomyces dermatitidis, Histoplasma capsulatum, Coccidioides spp., Paracoccidioides brasiliensis, Penicillium marneffei, and Sporothrix schenckii). Itraconazole is indicated for treatment of non-CNS-invasive aspergillosis[115] and increases response rates when added to steroid-treated patients with allergic bronchopulmonary aspergillosis.[116] Itraconazole capsules are the treatment of choice for indolent cases of blastomycosis and histoplasmosis,[117] nonmeningeal forms of coccidioidomycosis,[118] and paracoccidioidomycosis.[119] The capsule form is also useful for the treatment of sporotrichosis and penicilliosis marneffei (although not an approved indication) and the various dermatophytoses, including onychomycosis of the toenail and fingernail ( Table 5 ). HIV patients with histoplasmosis and penicilliosis should receive lifelong maintenance therapy. Itraconazole has no place in the therapy of cryptococcosis, mainly due to poor CNS penetration and high incidence of relapse. Intravenous itraconazole has been approved for use in persistently febrile neutropenic patients when a fungal etiology is suspected. This was based on an open randomized, controlled, multicenter trial in which intravenous itraconazole (followed by oral) was compared with conventional amphotericin B and was found to have equivalent efficacy in a relatively low-risk population and had less toxicity.[120] The oral itraconazole solution is indicated for the therapy of oropharyngeal and esophageal candidiasis. Finally, itraconazole suspension prevents invasive fungal infections when used for prophylaxis in patients with hematological malignancies [121] and can be a reasonable alternative to fluconazole in this setting. Voriconazole Voriconazole is active against most Candida and Aspergillus spp. Like itraconazole, voriconazole has significant activity against Scedosporium apiospermum (Pseudallescheria boydii) and is also active against fusariosis. Voriconazole is reasonably active in vitro against the endemic fungi (Blastomyces dermatitidis, Coccidioides spp., and Histoplasma capsulatum) as well as Cryptococcus neoformans, Trichosporon spp., and Acremonium kilensii. Clinical data about efficacy for these mycoses are insufficient. Voriconazole has been approved for the treatment of invasive aspergillosis since 2002 and has subsequently replaced amphotericin B for this indication. This conclusion has been based on a randomized, unblinded study of voriconazole versus amphotericin B deoxycholate for initial therapy of invasive aspergillosis.[90] In this study, starting treatment with voriconazole was more efficacious and safer than conventional amphotericin B (52.8% vs 31.6% successful outcomes). In a secondary end point analysis voriconazole offered better 12-week survival rates (70.8% vs 57.9%). In a second key study, voriconazole was compared with liposomal amphotericin B in a randomized multicenter trial as empirical antifungal treatment in febrile neutropenia.[88] Voriconazole marginally failed to meet the predefined margin for noninferiority and hence was not approved for this indication. However, voriconazole was safer than amphotericin B and was associated with fewer fungal breakthrough infections (1.9% vs 5%). The efficacy of voriconazole for the treatment of esophageal candidiasis compared with fluconazole was evaluated by a large, randomized, double-blind, multicenter trial in immunocompromised patients.[89] In this study the use of voriconazole at a dose of 200 mg twice daily was as effective as fluconazole but was associated with more adverse event-related discontinuation of treatment. This dose is also indicated for the treatment of candidemia and other deep Candida infections in nonneutropenic patients.[122] Responses of 45.5% and 30% have been reported for fusariosis and scedosporiosis, respectively,[123] with evidence of better results for Scedosporium apiospermum than S. prolificans.[124] This led to approval of voriconazole treatment as salvage therapy for these indications. Penicilliosis marneffei seems to respond as well.[123] Voriconazole has an extended spectrum of activity when compared with other azoles but no efficacy toward the agents of mucormycosis. This has led to a possible increase in cases of zygomycosis in voriconazole-treated patients; this possibility should be considered in cases of progressive fungal infections in patients undergoing voriconazole treatment, especially those in high-risk populations. Posaconazole Posaconazole has potent in vitro activity against most Candida and Aspergillus species. It is also active against Scedosporium spp., Fusarium spp., Histoplasma spp., Coccidioides spp., Penicillium marneffei, Sporothrix schenckii, Blastomyces dermatitidis, Trichosporon spp., and Cryptococcus neoformans as well as several dematiaceous molds. The most distinctive characteristic, though, when compared with other azoles, is the improved activity posaconazole exhibits against the zygomycetes. Posaconazole is approved for prophylaxis against fungal infections in severely immunocompromised patients, such as hematopoietic stem cell transplant patients (HSCT), who are at least 13 years of age, with graft versus host disease (GVHD) and patients with hematologic malignancies and neutropenia due to chemotherapy. This indication was based on two studies that compared posaconazole with fluconazole and fluconazole or itraconazole, respectively. The first was a randomized, multicenter, double-blind, and double-dummy study in HSCT patients with GVHD treated with either 200 mg posaconazole three times daily or fluconazole 400 mg daily.[101] The primary efficacy end point was the incidence of proven or probable fungal infections during the interval between randomization and day 112 in the intention to treat population. Posaconazole was as effective as fluconazole in prevention of invasive fungal infections, but posaconazole was superior to fluconazole in prevention of aspergillosis. The second study was a multicenter, randomized, unblinded study with evaluators unaware of the treatment assignments in patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome.[99] Patients received 200 mg posaconazole three times daily or either fluconazole suspension 400 mg daily or itraconazole solution 200 mg twice daily. The therapy was administered for the duration of neutropenia until occurrence of invasive fungal infection or for 12 weeks after randomization, whichever came first. The primary end point was occurrence of proven or probable fungal infection during prophylaxis in the intension-to-treat population. The use of galactomannan to diagnose most of the aspergillosis breakthrough cases remains a controversial feature of these two studies. With this reservation, posaconazole was superior to the comparator for the prevention of invasive fungal infections. Posaconazole is also approved for the treatment of patients with oropharyngeal candidiasis, including those refractory to fluconazole and itraconazole. The dose for this indication is 100 mg daily or up to 400 mg twice daily for refractory to other treatment patients. In the respective studies, posaconazole was as effective as fluconazole[100] in treatment-naive patients or exhibited a satisfactory response in refractory patients.[98] Finally, despite the fact that posaconazole has not received an indication for salvage therapy of refractory fungal infections, several uncontrolled case studies demonstrated a promising response rate in this setting for zygomycosis, histoplasmosis, fusariosis, coccidioidomycosis, or patients with chronic granulomatous disease.[102,125-130] Table 1. Triazole Formulations Triazole Oral Intravenous Fluconazole 50,100,150, and 200 mg tabs, oral suspension Yes Itraconazole 100 mg caps, oral solution* Yes† Voriconazole 50 and 200 mg tabs, oral suspension Yes Posaconazole Oral suspension No *Caps and oral solution are not bioequivalent and should not be used interchangeably. form recently withdrawn from US market. †Intravenous Table 2. Drugs With Increased Concentration When Coadministered With Azole Drug Fluconazole Voriconazole Itraconazole Posaconazole +[20] + Alprazolama + + Astemizole X +[21] Alfentanil +[19] Buspirone + + +[22] Busulphan -[23] + +[23] Caffeine Carbamazepine Cisapride Cyclophosphamide Cyclosporine + +[24] X + X -[25] +[26,27] Digoxin X +[28,29] +[28,30] -[32] +[30,33] + Dofetilide + Efavirenz X + + Eplerenone + + X X Erlotinib + Eszopiclone + + + Gefitinib +[36] +[37] +[38] +[38] +[39] Imatinib Irinotecan Losartan (+) + Haloperidol Ibuprofen +[34] (X) +[35] Fexofenadine Glimepiridec] +[31] + Eletriptan Ergot alkaloids (X) +[25] Docetaxel Felodipineb] (+) + +[40] -[40] Lovastatind + + X[41] Methadone +[42] +[43] + (+) Methylprednisolonee + + +[44] Midazolama +[45] +[46] X[45] Nevirapine + -[47] +[48] Omeprazole +[49] Paroxetine Phenytoin +[50] Pimozide Quinidine + Ramelteon + +[51] -[52] +[53] X X (X) X X[54] (X) + Ranolazine Rifabutin + +[55] X Risperidonef Saquinavirg + +[58] + X + Solifenacin + Sunitinib + +[26] Terfenadine Triazolama +[56] +[57] Sirolimus Tacrolimus + +[62] +[59] + +[60] X +[61] (+) + X[63] + + (+) +[65] Valdenafilh Vincristinei + +[64] Vinorelbine + +[66] +[68] +[69] +[72] +[71] Warfarinj +[67] Zidovudine +[70] Zolpidem +[71] (+) + = interaction; X = contraindication; - = absence of interaction. aIncludes most benzodiazepines except: bromazepam, diazepam, temazepam, and estazolam. bIncludes most Ca channel inhibitors. cIncludes glyburide and tolbutamide. dIncludes simvastatin and atorvastatin but not pravastatin, fluvastatin, and rosuvastatin. eIncludes betamethasone, dexamethasone, hydrocortisone, fludrocortisone, budesonide, and fluticasone, but not prednisolone. fIncludes ziprasidone. gPossibly includes all protease inhibitors. Fluconazole does not affect indinavir and ritonavir. hIncludes tadalafil, sildenafil. iIncludes vinblastin. jIncludes acenocoumarol. Table 3. Drugs That Decrease Azole Concentration When Coadministered Drug Fluconazole Voriconazole Itraconazole Posaconazole + -[73] + + Cimetidine + + Didanoside +[74] Antacids - Carbamazepine + X Efavirenz X + Grapefruit juice - - +[75] H2 inhibitors - -[76] +[77] Nevirapine + +[47] Phenobarbital X + -[50] +[51] +[52] +[53] - -[48] +[78] - +[79] X + +[56] Rifabutin X + + Ritonavir X Phenytoin Proton pump inhibitor Rifampin St. John's wort Sucralfate +[80] + - + - + = interaction; X = contraindication; - = absence of interaction. Table 4. Inhibition of CYP450 Enzymes by Triazoles Triazole CYP3A4 CYP2C9 CYP2C19 Fluconazole Moderate Strong Moderate Itraconazole Strong - - Voriconazole Moderate Moderate Weak Posaconazole Moderate - - - = no inhibition. Table 5. Approved Indications and Doses for Triazoles in the United States Triazole Fluconazole Indication Dose Vaginal candidiasis 150 mg one time dose Oropharyngeal and esophageal candidiasis 200 mg the first day, then 100 mg daily Invasive candidiasis, including candidemia in nonneutropenic patients 400 mg IV q24h then 400 mg PO daily Urinary Candida infections 50-200 mg daily Cryptococcosis 400 mg daily Prophylaxis of HSCT patients treated 400 mg daily with cytotoxic chemotherapy and/or radiation Itraconazole Aspergillosis 200 mg twice daily for 2 days followed by 200 mg IV daily for 2 wks then 400 mg PO daily Blastomycosis Histoplasmosis Empirical therapy in febrile neutropenia Oropharyngeal candidiasis 200 mg daily (solution) Esophageal candidiasis 100-200 mg daily (solution) Onychomycosis (fingernail) 200 mg twice daily for 1 week; two courses 1 month apart (capsule) Onychomycosis (toenail) 200 mg once daily for 12 weeks (capsule) Voriconazole Invasive aspergillosis Scedosporiosis 6 mg/kg twice daily for 1 day, followed by 4 mg/kg twice daily (intravenous), then 200 mg twice daily (oral) Fusariosis Candidemia or deep Candida infections in nonneutropenic patients Esophageal candidiasis 200 mg twice daily Posaconazole Prophylaxis against invasive aspergillosis or candidiasis in severely immunocompromised patients 200 mg three times daily Oropharyngeal candidiasis 100 mg twice daily (first day loading dose), then 100 mg daily Treatment failure or oropharyngeal candidiasis refractory to fluconazole or itraconazole 400 mg twice daily HSCT = hematopoietic stem cell transplantation. Table 6. Daily Cost of Oral Azole Treatment*,† Triazole Daily Dose Cost Fluconazole 200 mg $17 ($2 generic) Itraconazole 200 mg (solution) $21 Voriconazole 400 mg $78 Posaconazole 800 mg $110 *2007 †At Red Book (average wholesale price). doses recommended for treatment of esophageal candidiasis. References 1. Brammer KW, Farrow PR, Faulkner JK. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev Infect Dis 1990; 12(Suppl 3): S318S326 2. Barone JA, Moskovitz BL, Guarnieri J. Food interaction and steady-state pharmacokinetics of itraconazole oral solution in healthy volunteers. Pharmacotherapy 1998; 18: 295-301 3. Barone JA, Moskovitz BL, Guarnieri J. Enhanced bioavailability of itraconazole in hydroxypropyl-beta-cyclodextrin solution versus capsules in healthy volunteers. Antimicrob Agents Chemother 1998; 42: 1862-1865 4. Kintzel PE, Rollins CJ, Yee WJ, List AF. Low itraconazole serum concentrations following administration of itraconazole suspension to critically ill allogeneic bone marrow transplant recipients. Ann Pharmacother 1995; 29: 140-143 5. Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos 2003; 31: 540547 6. Hariprasad SM, Mieler WF, Holz ER. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol 2004; 122: 42-47 7. Roffey SJ, Cole S, Comby P. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab Dispos 2003; 31: 731-741 8. Boyd AE, Modi S, Howard SJ, Moore CB, Keevil BG, Denning DW. Adverse reactions to voriconazole. Clin Infect Dis 2004; 39: 1241-1244 9. Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J Clin Pharmacol 2006; 46: 235-243 10. Imhof A, Schaer DJ, Schanz U, Schwarz U. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med Wkly 2006; 136: 739-742 11. Smith J, Safdar N, Knasinski V. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother 2006; 50: 1570-1572 12. Trifilio S, Pennick G, Pi J. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer 2007; 109: 1532-1535 13. Ikeda Y, Umemura K, Kondo K, Sekiguchi K, Miyoshi S, Nakashima M. Pharmacokinetics of voriconazole and cytochrome P450 2C19 genetic status. Clin Pharmacol Ther 2004; 75: 587-588 14. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev 2003; 35: 99-106 15. Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol 2004; 57: 218-222 16. Ezzet F, Wexler D, Courtney R, Krishna G, Lim J, Laughlin M. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin Pharmacokinet 2005; 44: 211-220 17. Ullmann AJ, Cornely OA, Burchardt A. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob Agents Chemother 2006; 50: 658-666 18. Wexler D, Courtney R, Richards W, Banfield C, Lim J, Laughlin M. Effect of posaconazole on cytochrome P450 enzymes: a randomized, open-label, two-way crossover study. Eur J Pharm Sci 2004; 21: 645-653 19. Palkama VJ, Isohanni MH, Neuvonen PJ, Olkkola KT. The effect of intravenous and oral fluconazole on the pharmacokinetics and pharmacodynamics of intravenous alfentanil. Anesth Analg 1998; 87: 190-194 20. Saari TI, Laine K, Leino K, Valtonen M, Neuvonen PJ, Olkkola KT. Voriconazole, but not terbinafine, markedly reduces alfentanil clearance and prolongs its half-life. Clin Pharmacol Ther 2006; 80: 502-508 21. Lefebvre RA, Van Peer A, Woestenborghs R. Influence of itraconazole on the pharmacokinetics and electrocardiographic effects of astemizole. Br J Clin Pharmacol 1997; 43: 319-322 22. Kivisto KT, Lamberg TS, Kantola T, Neuvonen PJ. Plasma buspirone concentrations are greatly increased by erythromycin and itraconazole. Clin Pharmacol Ther 1997; 62: 348-354 23. Buggia I, Zecca M, Alessandrino EP. Itraconazole can increase systemic exposure to busulfan in patients given bone marrow transplantation. GITMO (Gruppo Italiano Trapianto di Midollo Osseo). Anticancer Res 1996; 16: 20832088 24. Nair DR, Morris HH. Potential fluconazole-induced carbamazepine toxicity. Ann Pharmacother 1999; 33: 790-792 25. Marr KA, Leisenring W, Crippa F. Cyclophosphamide metabolism is affected by azole antifungals. Blood 2004; 103: 1557-1559 26. Osowski CL, Dix SP, Lin LS, Mullins RE, Geller RB, Wingard JR. Evaluation of the drug interaction between intravenous high-dose fluconazole and cyclosporine or tacrolimus in bone marrow transplant patients. Transplantation 1996; 61: 1268-1272 27. Lopez-Gil JA. Fluconazole-cyclosporine interaction: a dose-dependent effect?. Ann Pharmacother 1993; 27: 427-430 28. Saad AH, DePestel DD, Carver PL. Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy 2006; 26: 1730-1744 29. Romero AJ, Pogamp PL, Nilsson LG, Wood N. Effect of voriconazole on the pharmacokinetics of cyclosporine in renal transplant patients. Clin Pharmacol Ther 2002; 71: 226-234 30. Partanen J, Jalava KM, Neuvonen PJ. Itraconazole increases serum digoxin concentration. Pharmacol Toxicol 1996; 79: 274-276 31. Courtney RDSP, Laughlin M, Lim J. Effect of posaconazole on the pharmacokinetics of cyclosporine. American Society for Microbiology Washington, DC: 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, December 16-19, 2001 2001, p. A27 32. Purkins L, Wood N, Kleinermans D, Nichols D. Voriconazole does not affect the steady-state pharmacokinetics of digoxin. Br J Clin Pharmacol 2003; 56(Suppl 1): 45-50 33. Jalava KM, Partanen J, Neuvonen PJ. Itraconazole decreases renal clearance of digoxin. Ther Drug Monit 1997; 19: 609-613 34. Jalava KM, Olkkola KT, Neuvonen PJ. Itraconazole greatly increases plasma concentrations and effects of felodipine. Clin Pharmacol Ther 1997; 61: 410-415 35. Shimizu M, Uno T, Sugawara K, Tateishi T. Effects of single and multiple doses of itraconazole on the pharmacokinetics of fexofenadine, a substrate of Pglycoprotein. Br J Clin Pharmacol 2006; 62: 372-376 36. Niemi M, Backman JT, Neuvonen M, Laitila J, Neuvonen PJ, Kivisto KT. Effects of fluconazole and fluvoxamine on the pharmacokinetics and pharmacodynamics of glimepiride. Clin Pharmacol Ther 2001; 69: 194-200 37. Yasui N, Kondo T, Otani K. Effects of itraconazole on the steady-state plasma concentrations of haloperidol and its reduced metabolite in schizophrenic patients: in vivo evidence of the involvement of CYP3A4 for haloperidol metabolism. J Clin Psychopharmacol 1999; 19: 149-154 38. Hynninen VV, Olkkola KT, Leino K. Effects of the antifungals voriconazole and fluconazole on the pharmacokinetics of s-( + )- and R-(-)Ibuprofen. Antimicrob Agents Chemother 2006; 50: 1967-1972 39. Dutreix C, Peng B, Mehring G. Pharmacokinetic interaction between ketoconazole and imatinib mesylate (Glivec) in healthy subjects. Cancer Chemother Pharmacol 2004; 54: 290-294 40. Kaukonen KM, Olkkola KT, Neuvonen PJ. Fluconazole but not itraconazole decreases the metabolism of losartan to E-3174. Eur J Clin Pharmacol 1998; 53: 445-449 41. Kivisto KT, Kantola T, Neuvonen PJ. Different effects of itraconazole on the pharmacokinetics of fluvastatin and lovastatin. Br J Clin Pharmacol 1998; 46: 49-53 42. Tarumi Y, Pereira J, Watanabe S. Methadone and fluconazole: respiratory depression by drug interaction. J Pain Symptom Manage 2002; 23: 148-153 43. Liu P, Foster G, Labadie R, Somoza E, Sharma A. Pharmacokinetic interaction between voriconazole and methadone at steady state in patients on methadone therapy. Antimicrob Agents Chemother 2007; 51: 110-118 44. Varis T, Kaukonen KM, Kivisto KT, Neuvonen PJ. Plasma concentrations and effects of oral methylprednisolone are considerably increased by itraconazole. Clin Pharmacol Ther 1998; 64: 363-368 45. Olkkola KT, Ahonen J, Neuvonen PJ. The effects of the systemic antimycotics, itraconazole and fluconazole, on the pharmacokinetics and pharmacodynamics of intravenous and oral midazolam. Anesth Analg 1996; 82: 511-516 46. Saari TI, Laine K, Leino K, Valtonen M, Neuvonen PJ, Olkkola KT. Effect of voriconazole on the pharmacokinetics and pharmacodynamics of intravenous and oral midazolam. Clin Pharmacol Ther 2006; 79: 362-370 47. Jaruratanasirikul S, Sriwiriyajan S. Pharmacokinetic study of the interaction between itraconazole and nevirapine. Eur J Clin Pharmacol 2007; 63: 451-456 48. Wood N, Tan K, Purkins L. Effect of omeprazole on the steady-state pharmacokinetics of voriconazole. Br J Clin Pharmacol 2003; 56(Suppl 1): 5661 49. Yasui-Furukori N, Saito M, Niioka T, Inoue Y, Sato Y, Kaneko S. Effect of itraconazole on pharmacokinetics of paroxetine: the role of gut transporters. Ther Drug Monit 2007; 29: 45-48 50. Blum RA, Wilton JH, Hilligoss DM. Effect of fluconazole on the disposition of phenytoin. Clin Pharmacol Ther 1991; 49: 420-425 51. Purkins L, Wood N, Ghahramani P, Love ER, Eve MD, Fielding A. Coadministration of voriconazole and phenytoin: pharmacokinetic interaction, safety, and toleration. Br J Clin Pharmacol 2003; 56(Suppl 1): 37-44 52. Ducharme MP, Slaughter RL, Warbasse LH. Itraconazole and hydroxyitraconazole serum concentrations are reduced more than tenfold by phenytoin. Clin Pharmacol Ther 1995; 58: 617-624 53. Krishna G, Sansone-Parsons A, Kantesaria B. Drug interaction assessment following concomitant administration of posaconazole and phenytoin in healthy men. Curr Med Res Opin 2007; 23: 1415-1422 54. Kaukonen KM, Olkkola KT, Neuvonen PJ. Itraconazole increases plasma concentrations of quinidine. Clin Pharmacol Ther 1997; 62: 510-517 55. Trapnell CB, Narang PK, Li R, Lavelle JP. Increased plasma rifabutin levels with concomitant fluconazole therapy in HIV-infected patients. Ann Intern Med 1996; 124: 573-576 56. Krishna G, Parsons A, Kantesaria B, Mant T. Evaluation of the pharmacokinetics of posaconazole and rifabutin following co-administration to healthy men. Curr Med Res Opin 2007; 23: 545-552 57. Jung SM, Kim KA, Cho HK. Cytochrome P450 3A inhibitor itraconazole affects plasma concentrations of risperidone and 9-hydroxyrisperidone in schizophrenic patients. Clin Pharmacol Ther 2005; 78: 520-528 58. Koks CH, Crommentuyn KM, Hoetelmans RM. The effect of fluconazole on ritonavir and saquinavir pharmacokinetics in HIV-1-infected individuals. Br J Clin Pharmacol 2001; 51: 631-635 59. Pai MP, Allen S. Voriconazole inhibition of tacrolimus metabolism. Clin Infect Dis 2003; 36: 1089-1091 60. Sansone ABD, Bella D, Statkevich P. Effect of posaconazole on the pharmacokinetics of tacrolimus in healthy volunteers. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Illinois, September 14-17, 2003. American Society for Microbiology Washington, DC: 2003, p. 1603 61. Pohjola-Sintonen SS, Vitasoto M, Toivenen L. Itraconazole prevents terfenadine metabolism and increases risk of torsades de pointes ventricular tachycardia. Eur J Clin Pharmacol 1993; 45: 191-193 62. Varhe A, Olkkola KT, Neuvonen PJ. Effect of fluconazole dose on the extent of fluconazole-triazolam interaction. Br J Clin Pharmacol 1996; 42: 465-470 63. Neuvonen PJ, Varhe A, Olkkola KT. The effect of ingestion time interval on the interaction between itraconazole and triazolam. Clin Pharmacol Ther 1996; 60: 326-331 64. Bermudez M, Fuster JL, Llinares E, Galera A, Gonzalez C. Itraconazole-related increased vincristine neurotoxicity: case report and review of literature. J Pediatr Hematol Oncol 2005; 27: 389-392 65. Mantadakis E, Amoiridis G, Kondi A, Kalmanti M. Possible increase of the neurotoxicity of vincristine by the concurrent use of posaconazole in a young adult with leukemia. J Pediatr Hematol Oncol 2007; 29: 130 66. Bosque E. Possible drug interaction between itraconazole and vinorelbine tartrate leading to death after one dose of chemotherapy. Ann Intern Med 2001; 134: 427 67. Black DJ, Kunze KL, Wienkers LC. Warfarin-fluconazole, II: A metabolically based drug interaction: in vivo studies. Drug Metab Dispos 1996; 24: 422-428 68. Purkins L, Wood N, Kleinermans D, Nichols D. Voriconazole potentiates warfarin-induced prothrombin time prolongation. Br J Clin Pharmacol 2003; 56(Suppl 1): 24-29 69. Yeh J, Soo SC, Summerton C, Richardson C. Potentiation of action of warfarin by itraconazole. BMJ 1990; 301: 669 70. Sahai J, Gallicano K, Pakuts A, Cameron DW. Effect of fluconazole on zidovudine pharmacokinetics in patients infected with human immunodeficiency virus. J Infect Dis 1994; 169: 1103-1107 71. Greenblatt DJ, von Moltke LL, Harmatz JS. Kinetic and dynamic interaction study of zolpidem with ketoconazole, itraconazole, and fluconazole. Clin Pharmacol Ther 1998; 64: 661-671 72. Saari TI, Laine K, Leino K, Valtonen M, Neuvonen PJ, Olkkola KT. Effect of voriconazole on the pharmacokinetics and pharmacodynamics of zolpidem in healthy subjects. Br J Clin Pharmacol 2007; 63: 116-120 73. Courtney R, Radwanski E, Lim J, Laughlin M. Pharmacokinetics of posaconazole coadministered with antacid in fasting or nonfasting healthy men. Antimicrob Agents Chemother 2004; 48: 804-808 74. May DB, Drew RH, Yedinak KC, Bartlett JA. Effect of simultaneous didanosine administration on itraconazole absorption in healthy volunteers. Pharmacotherapy 1994; 14: 509-513 75. Penzak SR, Gubbins PO, Gurley BJ, Wang PL, Saccente M. Grapefruit juice decreases the systemic availability of itraconazole capsules in healthy volunteers. Ther Drug Monit 1999; 21: 304-309 76. Purkins L, Wood N, Kleinermans D, Nichols D. Histamine H2-receptor antagonists have no clinically significant effect on the steady-state pharmacokinetics of voriconazole. Br J Clin Pharmacol 2003; 56(Suppl 1): 5155 77. Lim SG, Sawyerr AM, Hudson M, Sercombe J, Pounder RE. Short report: the absorption of fluconazole and itraconazole under conditions of low intragastric acidity. Aliment Pharmacol Ther 1993; 7: 317-321 78. Jaruratanasirikul S, Sriwiriyajan S. Effect of omeprazole on the pharmacokinetics of itraconazole. Eur J Clin Pharmacol 1998; 54: 159-161 79. Panomvana Na Ayudhya D, Thanompuangseree N, Tansuphaswadikul S. Effect of rifampicin on the pharmacokinetics of fluconazole in patients with AIDS. Clin Pharmacokinet 2004; 43: 725-732 80. Rengelshausen J, Banfield M, Riedel KD. Opposite effects of short-term and long-term St John's wort intake on voriconazole pharmacokinetics. Clin Pharmacol Ther 2005; 78: 25-33 81. Wang EJ, Lew K, Casciano CN, Clement RP, Johnson WW. Interaction of common azole antifungals with P glycoprotein. Antimicrob Agents Chemother 2002; 46: 160-165 82. Stevens DA, Diaz M, Negroni R. Safety evaluation of chronic fluconazole therapy. Fluconazole Pan-American Study Group. Chemotherapy 1997; 43: 371377 83. Pappas PG, Kauffman CA, Perfect J. Alopecia associated with fluconazole therapy. Ann Intern Med 1995; 123: 354-357 84. Pursley TJ, Blomquist IK, Abraham J, Andersen HF, Bartley JA. Fluconazoleinduced congenital anomalies in three infants. Clin Infect Dis 1996; 22: 336-340 85. Tucker RM, Haq Y, Denning DW, Stevens DA. Adverse events associated with itraconazole in 189 patients on chronic therapy. J Antimicrob Chemother 1990; 26: 561-566 86. Sharkey PK, Rinaldi MG, Dunn JF, Hardin TC, Fetchick RJ, Graybill JR. Highdose itraconazole in the treatment of severe mycoses. Antimicrob Agents Chemother 1991; 35: 707-713 87. Ahmad SR, Singer SJ, Leissa BG. Congestive heart failure associated with itraconazole. Lancet 2001; 357: 1766-1767 88. Walsh TJ, Pappas P, Winston DJ. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med 2002; 346: 225-234 89. Ally R, Schurmann D, Kreisel W. A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients. Clin Infect Dis 2001; 33: 14471454 90. Herbrecht R, Denning DW, Patterson TF. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002; 347: 408-415 91. Scherpbier HJ, Hilhorst MI, Kuijpers TW. Liver failure in a child receiving highly active antiretroviral therapy and voriconazole. Clin Infect Dis 2003; 37: 828-830 92. Vandecasteele SJ, Van Wijngaerden E, Peetermans WE. Two cases of severe phototoxic reactions related to long-term outpatient treatment with voriconazole. Eur J Clin Microbiol Infect Dis 2004; 23: 656-657 93. Curigliano G, Formica V, De Pas T. Life-threatening toxic epidermal necrolysis during voriconazole therapy for invasive aspergillosis after chemotherapy. Ann Oncol 2006; 17: 1174-1175 94. McCarthy KL, Playford EG, Looke DF, Whitby M. Severe photosensitivity causing multifocal squamous cell carcinomas secondary to prolonged voriconazole therapy. Clin Infect Dis 2007; 44: e55-e56 95. Tolland JP, McKeown PP, Corbett JR. Voriconazole-induced pseudoporphyria. Photodermatol Photoimmunol Photomed 2007; 23: 29-31 96. Philips JA, Marty FM, Stone RM, Koplan BA, Katz JT, Baden LR. Torsades de pointes associated with voriconazole use. Transpl Infect Dis 2007; 9: 33-36 97. Tsiodras S, Zafiropoulou R, Kanta E, Demponeras C, Karandreas N, Manesis EK. Painful peripheral neuropathy associated with voriconazole use. Arch Neurol 2005; 62: 144-146 98. Skiest DJ, Vazquez JA, Anstead GM. Posaconazole for the treatment of azolerefractory oropharyngeal and esophageal candidiasis in subjects with HIV infection. Clin Infect Dis 2007; 44: 607-614 99. Cornely OA, Maertens J, Winston DJ. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356: 348-359 100. Vazquez JA, Skiest DJ, Nieto L. A multicenter randomized trial evaluating posaconazole versus fluconazole for the treatment of oropharyngeal candidiasis in subjects with HIV/AIDS. Clin Infect Dis 2006; 42: 1179-1186 101. Ullmann AJ, Lipton JH, Vesole DH. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007; 356: 335347 102. Walsh TJ, Raad I, Patterson TF. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis 2007; 44: 2-12 103. Raad II, Graybill JR, Bustamante AB. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis 2006; 42: 1726-1734 104. Rex JH, Bennett JE, Sugar AM. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med 1994; 331: 1325-1330 105. Rex JH, Pappas PG, Karchmer AW. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis 2003; 36: 1221-1228 106. Fluckiger U, Marchetti O, Bille J. Treatment options of invasive fungal infections in adults. Swiss Med Wkly 2006; 136: 447-463 107. Slavin MA, Osborne B, Adams R. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation: a prospective, randomized, double-blind study. J Infect Dis 1995; 171: 1545-1552 108. Goodman JL, Winston DJ, Greenfield RA. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992; 326: 845-851 109. MacMillan ML, Goodman JL, DeFor TE, Weisdorf DJ. Fluconazole to prevent yeast infections in bone marrow transplantation patients: a randomized trial of high versus reduced dose, and determination of the value of maintenance therapy. Am J Med 2002; 112: 369-379 110. Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med 2001; 345: 1660-1666 111. Manzoni P, Stolfi I, Pugni L. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med 2007; 356: 24832495 112. Mussini C, Pezzotti P, Miro JM. Discontinuation of maintenance therapy for cryptococcal meningitis in patients with AIDS treated with highly active antiretroviral therapy: an international observational study. Clin Infect Dis 2004; 38: 565-571 113. Saag MS, Powderly WG, Cloud GA. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med 1992; 326: 83-89 114. Galgiani JN, Catanzaro A, Cloud GA. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis: a randomized, double-blind trial. Mycoses Study Group. Ann Intern Med 2000; 133: 676-686 115. Denning DW, Lee JY, Hostetler JS. NIAID Mycoses Study Group Multicenter Trial of Oral Itraconazole Therapy for Invasive Aspe rgillosis. Am J Med 1994; 97: 135-144 116. Stevens DA, Schwartz HJ, Lee JY. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med 2000; 342: 756-762 117. Dismukes WE, Bradsher RW, Cloud GC. Itraconazole therapy for blastomycosis and histoplasmosis. NIAID Mycoses Study Group. Am J Med 1992; 93: 489-497 118. Graybill JR, Stevens DA, Galgiani JN, Dismukes WE, Cloud GA. Itraconazole treatment of coccidioidomycosis. NAIAD Mycoses Study Group. Am J Med 1990; 89: 282-290 119. Naranjo MS, Trujillo M, Munera MI, Restrepo P, Gomez I, Restrepo A. Treatment of paracoccidioidomycosis with itraconazole. J Med Vet Mycol 1990; 28: 67-76 120. Boogaerts M, Winston DJ, Bow EJ. Intravenous and oral itraconazole versus intravenous amphotericin B deoxycholate as empirical antifungal therapy for persistent fever in neutropenic patients with cancer who are receiving broadspectrum antibacterial therapy: a randomized, controlled trial. Ann Intern Med 2001; 135: 412-422 121. Glasmacher A, Prentice A, Gorschluter M. Itraconazole prevents invasive fungal infections in neutropenic patients treated for hematologic malignancies: evidence from a meta-analysis of 3,597 patients. J Clin Oncol 2003; 21: 46154626 122. Kullberg BJ, Sobel JD, Ruhnke M. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 2005; 366: 1435-1442 123. Perfect JR, Marr KA, Walsh TJ. Voriconazole treatment for lesscommon, emerging, or refractory fungal infections. Clin Infect Dis 2003; 36: 1122-1131 124. Torre-Cisneros JGRA, Hodges MR, Lutsar I. Voriconazole (VORI) for the treatment of S. apiospermum and S. prolificans infection. New Orleans, LA: Programs and abstracts of the 38th Annual Meeting of the Infectious Diseases Society of America September 7-10, 2000 125. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis 2006; 42: e61-e65 126. Greenberg RN, Mullane K, van Burik JA. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother 2006; 50: 126-133 127. Restrepo A, Tobon A, Clark B. Salvage treatment of histoplasmosis with posaconazole. J Infect 2007; 54: 319-327 128. Raad II, Hachem RY, Herbrecht R. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin Infect Dis 2006; 42: 1398-1403 129. Anstead GM, Corcoran G, Lewis J, Berg D, Graybill JR. Refractory coccidioidomycosis treated with posaconazole. Clin Infect Dis 2005; 40: 17701776 130. Segal BH, Barnhart LA, Anderson VL, Walsh TJ, Malech HL, Holland SM. Posaconazole as salvage therapy in patients with chronic granulomatous disease and invasive filamentous fungal infection. Clin Infect Dis 2005; 40: 1684-1688 Funding Information This work has been funded by the Intramural Research Program of NIAID. Reprint Address John E Bennett, MD, 9000 Rockville Pike, Bldg. 10/11N234, Bethesda, MD 20892. Email: jbennett@niaid.nih.gov Dimitrios I. Zonios, John E. Bennett, Clinical Mycology Section, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Bethesda, Maryland