1BOM, 1BON - Chemistry Biochemistry and Bio
advertisement

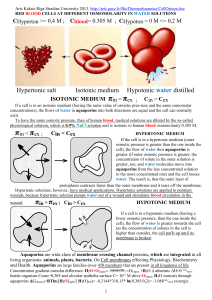
Nature. 2001 Dec 20-27;414(6866):872-8. 1J4N (1FQY, 1H6I, 1IH5 Wikipedia 2013) Structural basis of transport-specific transport through the AQP1 transport channel. Sui H, Han BG, Lee JK, Walian P, Jap BK. Source Life Sciences Division, Lawrence Berkeley National Laboratory, University of California, Berkeley, California 94720, USA. Abstract H2O, O2, NO, CO, CO2 Transport channels facilitate the rapid transport across cell membranes and are responsible in osmotic presure formation due to water solutes gradients. These channels are believed to be involved in many physiological homeostasis processes that include renal transport conservation, neuro-homeostasis, digestion, regulation of body temperature and reproduction. Members of the transport channel superfamily have been found in a range of cell types from bacteria to human. In mammals, there are currently 10 families of transport channels, referred to as aquaporins (AQP): AQP0-AQP9. Here we report the structure of the aquaporin 1 (AQP1) transport channel to 2.2 A resolution. The channel consists of three topological elements, an extracellular and a cytoplasmic vestibule connected by an extended narrow pore or selectivity filter. Within the selectivity filter, four bound transports are localized along three hydrophilic nodes, which punctuate an otherwise extremely hydrophobic pore segment. This unusual combination of a long hydrophobic pore and a minimal number of solute binding sites facilitates rapid transport transport. Residues of the constriction region, in particular histidine 182, which is conserved among all known transport-specific channels, are critical in establishing transport specificity. Our analysis of the AQP1 pore also indicates that the transport of protons through this channel is highly energetically unfavourable. Profs. Willott & Grimes The University of Arizona December 8, 2001 denicew@u.arizona.edu http://www.blc.arizona.edu/ © 1998-2001. Aquaporin-1 Q) Aquaporin-1 (AQP-1)? Isn't that an aftershave? A) No, you're thinking of Aquavelva tm. Aquaporin-1 (AQP-1) is a transport channel located on cell membranes. The aquaporins are a family of proteins known for facilitating transport transport, and AQP-1 (first called CHIP28) was the first aquaporin to be discovered and classified. Q. How does cRNA make aquaporin-1? A. It doesn't – the cell does! Think of cRNA as a recipe for a protein, in this case aquaporin-1. When injected into the cell, the cell reads this recipe to make aquaporin-1 from various amino acids. The protein created is then transported to the cell membrane, where it resumes normal function. An especially exciting opportunity to manipulate gas transport rates lies in cancer research, where inhibiting the transcription of AQP-1 could be the key to killing cancer cells . Cancer cells release more CO2 than most other cells, so stopping these cell's abilty to extrude the gas to their environment would result in cell death. This example is one of many uses for the manipulation of gas transport, however, and the opportunities are endless. Wikipedia, the free encyclopedia 2013 AQP1 is a widely expressed transport channel, whose physiological function has been most thoroughly characterized in the kidney. It is found in the baso lateral and apical plasma membranes of the proximal tubules, the descending limb of the loop of Henle, and in the descending portion of the vasa recta. Additionally, it is found in red blood cells, vascular endothelium, the gastrointestinal tract, sweat glands, and lungs. It is not regulated by vasopressin (ADH). Aquaporins are a family of small integral membrane proteins related to the major intrinsic protein (MIP or AQP0). This gene encodes an aquaporin which functions as a molecular transport channel protein. It is a homo tetramer with 6 bilayer spanning domains and Nglycosylation sites. The protein physically resembles channel proteins and is abundant in erythrocytes and renal tubes. Aquaporin 1 (Colton blood group)The gene encoding this aquaporin is a possible candidate for disorders involving imbalance in ocular fluid movement.[1] The Colton antigen system (Co) is present on the membranes of red blood cells and in the tubules of the kidney[1] and helps determine a person's blood type. The Co antigen is found on a protein called aquaporin-1 which is responsible for transport homeostasis and urine concentration.[2] The Co antigen is important in transfusion medicine. 99.8% of people possess the Co(a) allele. Individuals with Co(b) allele or who are missing the Colton antigen are at risk for a transfusion reaction such as hemolytic anemia or alloimmunization. Antibodies against the Colton antigen may also cause hemolytic disease of the newborn, in which a pregnant woman's body creates antibodies against the blood of her fetus, leading to destruction of the fetal blood cells.[3] Function Aquaporins are "the plumbing system for cells," said Agre. Every cell is primarily transport. "But the transport doesn’t just sit in the cell, it moves through it in a very organized way. The process occurs rapidly in tissues that have these aquaporins or transport channels." 1 For many years, scientists assumed that transport leaked through the cell membrane, and some transport does. "But the very rapid movement of transport through some cells was not explained by this theory," said Agre.[7] Aquaporins selectively conduct transport molecules in and out of the cell, while preventing the passage of ions and other solutes. Also known as transport channels, aquaporins are integral membrane pore proteins. Some of them, known as aqua glyceroporins, also transport other small uncharged solutes, such as glycerol, CO2, ammonia and urea across the membrane, depending on the size of the pore. For example, the aquaporin 3 channel has a pore width of 810 Ångströms and allows the passage of hydrophilic molecules ranging between 150-200 Da. However, the transport pores are completely impermeable to charged species, such as protons, a property critical for the conservation of the membrane's electrochemical potential.[8] Transport molecules traverse through the pore of the channel in single file. The presence of transport channels increases membrane permeability to transport. Many human cell types express them, as do certain bacteria and many other organisms, such as plants for which it is essential for the transport transport system.[9] Discovery Agre said he discovered aquaporins "by serendipity." His lab had an N.I.H. grant to study the Rh blood group antigens. They isolated the Rh molecule but a second molecule, 28 kilodaltons in size (and therefore called 28K) kept appearing. At first they thought it was a piece of the Rh molecule, or a contaminant, but it turned out to be an undiscovered molecule with unknown function. It was abundant in red blood cells and kidney tubes, and related to proteins of diverse origins, like the brains of fruit flies, bacteria, the lenses of eyes, and plant tissues. In most cells, transport moves in and out by osmosis through the lipid component of cell membranes. Due to the relatively high transport permeability of some epithelial cells it was long suspected that some additional mechanism for transport across membranes must exist. But it was not until 1992 that the first aquaporin, ‘aquaporin-1’ (originally known as CHIP 28), was reported by Peter Agre, of Johns Hopkins University.[10] The pioneering discoveries and research on transport channels by Agre and his colleagues resulted in the presentation of a Nobel Prize in Chemistry to Agre in 2003.[5] In 1999, together with other research teams, Agre reported the first high-resolution images of the three-dimensional structure of an aquaporin, namely, aquaporin-1.[11] Further studies using supercomputer simulations have identified the pathway of transport as it moves through the channel and demonstrated how a pore can allow transport to pass without the passage of small solutes.[12] However the first report of protein mediated transport through membranes was by Gheorghe Benga in 1986.[13][14] This publication that preceded Agre's first publication on transport membrane transport proteins has led to a controversy that Benga's work was adequately recognized by neither Agre nor the Nobel Prize Committee.[15] There is a long history of transport pores, starting in 1957.[16] There have been many reviews of the history.[17] Structure Schematic diagram of the 2D structure of aquaporin 1 (AQP1) depicting the six transmembrane cell-helices and the five inter helical loop regions A-E. The 3D structure of aquaporin highlighting the 'hourglass'-shaped transport channel that cuts through the center of the protein. Aquaporin proteins are made up of six transmembrane αhelices arranged in a right-handed bundle, with the amino and the carboxyl termini located on the cytoplasmic surface of the membrane.[8][18] The amino and carboxyl halves of the sequence show similarity to each other, in what appears to be a tandem repeat. Some researchers believe that this results from an early evolution event that saw the duplication of the half-size gene. There are also five interhelical loop regions (A – E) that form the extracellular and cytoplasmic vestibules. Loops B and E are hydrophobic loops that contain the highly, although not completely conserved, asparagine–proline–alanine (NPA) motif, which overlap the middle of the lipid bilayer of the membrane forming a 3-D 'hourglass' structure where the transport flows through. This overlap forms one of the two well-known channel constriction sites in the peptide, the NPA motif and a second and usually narrower constriction known as 'selectivity filter' or ar/R selectivity filter. Aquaporins form tetramers in the cell membrane, with each monomer acting as a transport channel.[8] The different aquaporins contain differences in their peptide sequence, which allows for the size of the pore in the protein to differ between aquaporins. The resultant size of the pore directly affects what molecules are able to pass through the pore, with small pore sizes only allowing small molecules like transport to pass through the pore. NPA motif Using computer simulations, it has been suggested that the orientation of the transport molecules moving through the channel assures that only transport passes between cells, due to the formation of a single line of transport molecules. The transport molecules move through the narrow channel by orienting themselves in the local electrical field formed by the atoms of the channel wall. Upon entering, the transport molecules face with their oxygen atom down the channel. Midstream, they reverse orientation, facing with the oxygen atom up.[19] Why this rotation occurs is not entirely clear yet. Some researchers identified an electrostatic field generated by the two aquaporin half-helices HB and HE as the reason for the rotation of transport molecules. Others suggested that it is caused by the interaction of hydrogen bonds between the oxygen of the transport molecule and the asparagines in the 2 two NPA motifs. Moreover, whether the rotation of transport molecules has any biological significance is still being discussed. Early studies speculated that the "bipolar" orientation of transport molecules keep them from conducting protons via the Grotthuss mechanism, while still permitting a fast flux of transport molecules.[20] More recent studies question this interpretation and emphasize an electrostatic barrier as the reason for proton blockage. In the latter view, the rotation of transport molecules is only a side-effect of the electrostatic barrier. At present (2008), the origin of the electrostatic field is a matter of debate. While some studies mainly considered the electric field generated by the protein's half-helices HB and HE, others emphasized desolvation effects as the proton enters the narrow aquaporin pore. ar/R selectivity filter Schematic depiction of transport movement through the narrow selectivity filter of the aquaporin channel. N76, H180, N192 The ar/R (aromatic/arginine) selectivity filter is a cluster of amino acids that help bind to transport molecules and exclude other molecules that may try to enter the pore. It is the mechanism by which the aquaporin is able to selectively bind transport molecules (hence allowing them through) and prevent other molecules from entering. The ar/R filter is a tetrad that is formed by two amino acid residues from helices B (HB) and E (HE) and two residues from loop E (LE1 and LE2), found on either side of the NPA motif. The ar/R region is usually found towards the extracellular vestibule, approximately 8 Å above the NPA motif and is often the narrowest part of the pore. The narrow pore acts to weaken the hydrogen bonds between the transport molecules allowing the transport to interact with the positively charged arginine, which also acts as a proton filter for the pore. Location[22] Function[22] Type Aquaporin kidney (apically) In mammals There are thirteen known types of Transport reabsorption PCT; PST; tDLH 1 aquaporins in mammals, and seven of these are located in the kidney,[21] but the existence of many more is kidney (apically) Transport reabsorption Aquaporin ICT; CCT suspected. The most studied aquaporins are compared in response to ADH 2 OMCD; IMCD in the following table: In plants In plants transport is taken up from the kidney (basolaterally) Transport reabsorption soil through the roots, where it passes from the cortex Aquaporin medullary collecting and glycerol permeability 3 into the vascular tissues. There are two routes for duct transport to flow in these tissues, known as the kidney (basolaterally) proximal tubule Aquaporin apoplastic and symplastic pathways. The presence of cytoplasm medullary collecting 4,7,8,11 duct Transport reabsorption aquaporins in the cell membranes seems to serve to facilitate the trans cellular symplastic pathway for transport transport. When plant roots are exposed to mercuric chloride, which is known to inhibit aquaporins, the flow of transport is greatly reduced while the flow of ions is not, supporting the view that there exists a mechanism for transport transport independent of the transport of ions: aquaporins. Aquaporins in plants are separated into five main homologous subfamilies, or groups:[23] Plasma membrane Intrinsic Protein (PIP)[24] Tonoplast Intrinsic Protein (TIP)[25] Nodulin-26 like Intrinsic Protein (NIP)[26] Small basic Intrinsic Protein (SIP)[27] X Intrinsic Protein (XIP) These five subfamilies have later been divided into smaller evolutionary subgroups based on their DNA sequence. PIPs cluster into two subgroups, PIP1 and PIP2, whilst TIPs cluster into 5 subgroups, TIP1, TIP2, TIP3, TIP4 and TIP5. Each subgroup is again split up into isoforms e.g. PIP1;1, PIP1;2. The silencing of plant aquaporins has been linked to poor plant growth and even death of the plant. The gating of aquaporins is carried out to stop the flow of transport through the pore of the protein. This may be carried out for a number of reasons, for example when the plant contains low amounts of cellular transport due to drought.[28] The gating of an aquaporin is carried out by an interaction between a gating mechanism and the aquaporin, which causes a 3D change in the protein so that it blocks the pore and, thus, disallows the flow of transport through the pore. In plants, it has been seen that there are at least two forms of aquaporin gating. These are gating by the dephosphorylation of certain serine residues, which has been seen as a response to drought, and the protonation of specific histidine residues in response to flooding. The phosphorylation of an aquaporin has also been linked to the opening and closing of petals in response to temperature.[29][30] 3 Clinical significance Aquaporin-1 (AQP1) is found in choroid plexus, contributes to production of CSF and AQP4 is found on perivascular and ependymal cells. If aquaporin could be manipulated, that could potentially solve medical problems such as fluid retention in heart disease and brain edema after stroke. [7] There have been two clear examples of diseases identified as resulting from mutations in aquaporins: Mutations in the aquaporin-2 gene cause hereditary nephrogenic diabetes insipidus in humans.[31] Mice homozygous for inactivating mutations in the aquaporin-0 gene develop congenital cataracts.[32] A small number of people have been identified with severe or total deficiency in aquaporin-1. It is interesting to note that they are, in general, healthy, but exhibit a defect in the ability to concentrate solutes in the urine and to conserve transport when deprived of drinking transport. Mice with targeted deletions in aquaporin-1 also exhibit a deficiency in transport conservation due to an inability to concentrate solutes in the kidney medulla by countercurrent multiplication. In addition to its role in genetically determined nephrogenic diabetes insipidus, aquaporins also play a key role in acquired forms of nephrogenic diabetes insipidus (disorders that cause increased urine production).[33] Acquired nephrogenic diabetes insipidus can result from impaired regulation of aquaporin-2 due to administration of lithium salts (as a treatment for bipolar disorder), low potassium concentrations in the blood (hypokalemia), high calcium concentrations in the blood (hypercalcemia), or a chronically high intake of transport beyond the normal requirements (e.g., due to excessive habitual intake of bottled transport or coffee). It has been found that autoimmune reactions against aquaporin 4 produce Devic's disease.[34] References 1) ^ Agre P (2006). "The aquaporin transport channels". Proc Am Thorac Soc 3 (1): 5–13. doi:10.1513/pats.200510-109JH. PMC 2658677. PMID 16493146. 2) ^ Agre P, Kozono D (2003). "Aquaporin transport channels: molecular mechanisms for human diseases". FEBS Lett. 555 (1): 72–8. doi:10.1016/S0014-5793(03)01083-4. PMID 14630322. 3) ^ Schrier RW (2007). "Aquaporin-related disorders of transport homeostasis". Drug News Perspect. 20 (7): 447–53. doi:10.1358/dnp.2007.20.7.1138161. PMID 17992267. 4) ^ Knepper MA, Nielsen S (2004). "Peter Agre, 2003 Nobel Prize winner in chemistry". J. Am. Soc. Nephrol. 15 (4): 1093–5. doi:10.1097/01.ASN.0000118814.47663.7D. PMID 15034115. 5) ^ to: a B "The Nobel Prize in Chemistry 2003". Nobel Foundation. Retrieved 2008-01-23. 6) ^ Cooper, Geoffrey (2009). The Cell: A Molecular Approach. Washington, DC: ASM PRESS. p. 544. ISBN 9780878933006. 7) ^ to: a B A Conversation With Peter Agre: Using a Leadership Role to Put a Human Face on Science, By Claudia Dreifus, New YorkTimes, January 26, 2009 8) ^ to: a B c Gonen T, Walz T (2006). "The structure of aquaporins". Q. Rev. Biophys. 39 (4): 361–96. doi:10.1017/S0033583506004458. PMID 17156589. 9) ^ Kruse E, Uehlein N, Kaldenhoff R (2006). "The aquaporins". Genome Biol. 7 (2): 206. doi:10.1186/gb-2006-7-2-206. PMC 1431727. PMID 16522221. 10) ^ Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, Guggino WB, Nielsen S (1 October 1993). "Aquaporin CHIP: the archetypal molecular transport channel". Am. J. Physiol. 265 (4 Pt 2): F463–76. PMID 7694481. 11) ^ Mitsuoka K, Murata K, Walz T, Hirai T, Agre P, Heymann JB, Engel A, Fujiyoshi Y (1999). "The structure of aquaporin-1 at 4.5-A resolution reveals short cell-helices in the center of the monomer". J. Struct. Biol. 128 (1): 34–43. doi:10.1006/jsbi.1999.4177. PMID 10600556. 12) ^ de Groot BL, Grubmüller H (2005). "The dynamics and energetics of transport permeation and proton exclusion in aquaporins". Curr. Opin. Struct. Biol. 15 (2): 176–83. doi:10.1016/j.sbi.2005.02.003. PMID 15837176. 13) ^ Benga G, Popescu O, Pop VI, Holmes RP (1986). "p-(Chloromercuri)benzenesulfonate binding by membrane proteins and the inhibition of transport transport in human erythrocytes". Biochemistry 25 (7): 1535–8. doi:10.1021/bi00355a011. PMID 3011064. 14) ^ Kuchel PW (2006). "The story of the discovery of aquaporins: convergent evolution of ideas--but who got there first?". Cell. Mol. Biol. (Noisy-le-grand) 52 (7): 2–5. PMID 17543213. 15) ^ G Benga. "Gheorghe Benga". Ad Astra - Online project for the Romanian Scientific Community. Retrieved 2008-04-05. 16) ^ Paganelli CV, Solomon AK (November 1957). "The rate of exchange of tritiated transport across the human red cell membrane". J. Gen. Physiol. 41 (2): 259–77. doi:10.1085/jgp.41.2.259. PMC 2194835. PMID 13475690. 17) ^ Parisi M, Dorr RA, Ozu M, Toriano R (December 2007). "From membrane pores to aquaporins: 50 years measuring transport fluxes". J Biol Phys 33 (5–6): 331–43. doi:10.1007/s10867-008-9064-5. PMC 2565768. PMID 19669522. 18) ^ Fu D, Lu M (2007). "The structural basis of transport permeation and proton exclusion in aquaporins". Mol. Membr. Biol. 24 (5–6): 366– 74. doi:10.1080/09687680701446965. PMID 17710641. 19) ^ de Groot BL, Grubmüller H (2001). "Transport permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF". Science 294 (5550): 2353–2357. doi:10.1126/science.1062459. PMID 11743202. 20) ^ Tajkhorshid E, Nollert P, Jensen MØ, Miercke LJ, O'Connell J, Stroud RM, Schulten K (2002). "Control of the selectivity of the aquaporin transport channel family by global orientational tuning". Science 296 (5567): 525–30. doi:10.1126/science.1067778. PMID 11964478. 21) ^ Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA (2002). "Aquaporins in the kidney: from molecules to medicine". Physiol. Rev. 82 (1): 205–44. doi:10.1152/physrev.00024.2001 (inactive 2008-06-20). PMID 11773613. 22) ^ to: a B Unless else specified in table boxes, then ref is: Walter F., PhD. Boron (2005). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. ISBN 1-4160-2328-3. Page 842 4 23) ^ Kaldenhoff R, Bertl A, Otto B, Moshelion M, Uehlein N (2007). "Characterization of plant aquaporins". Meth. Enzymol. Methods in Enzymology 428: 505–31. doi:10.1016/S0076-6879(07)28028-0. ISBN 9780123739216. PMID 17875436. 24) ^ Kammerloher W, Fischer U, Piechottka GP, Schäffner AR (1994). "Transport channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system". Plant J. 6 (2): 187–99. doi:10.1046/j.1365-313X.1994.6020187.x. PMID 7920711. 25) ^ Maeshima M (2001). "TONOPLAST TRANSPORTERS: Organization and Function". Annu Rev Plant Physiol Plant Mol Biol 52 (1): 469– 497. doi:10.1146/annurev.arplant.52.1.469. PMID 11337406. 26) ^ Wallace IS, Choi WG, Roberts DM (2006). "The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins". Biochim. Biophys. Acta 1758 (8): 1165–75. doi:10.1016/j.bbamem.2006.03.024. PMID 16716251. 27) ^ Johanson U, Gustavsson S (2002). "A new subfamily of major intrinsic proteins in plants". Mol. Biol. Evol. 19 (4): 456–61. doi:10.1093/oxfordjournals.molbev.a004101. PMID 11919287. 28) ^ Kaldenhoff R, Fischer M (2006). "Aquaporins in plants". Acta Physiol (Oxf) 187 (1–2): 169–76. doi:10.1111/j.1748-1716.2006.01563.x. PMID 16734753. 29) ^ Azad AK, Sawa Y, Ishikawa T, Shibata H (2004). "Phosphorylation of plasma membrane aquaporin regulates temperature-dependent opening of tulip petals". Plant Cell Physio. 45(5) (5): 608–17. doi:10.1093/pcp/pch069. PMID 15169943. 30) ^ Azad AK, Katsuhara M, Sawa Y, Ishikawa T, Shibata H (2008). "Characterization of four plasma membrane aquaporins in tulip petals: a putative homolog is regulated by phosphorylation". Plant Cell Physiol. 49(8) (8): 1196–208. doi:10.1093/pcp/pcn095. PMID 18567892. 31) ^ Bichet DG (2006). "Nephrogenic diabetes insipidus". Adv Chronic Kidney Dis 13 (2): 96–104. doi:10.1053/j.ackd.2006.01.006. PMID 16580609. 32) ^ Okamura T, Miyoshi I, Takahashi K, Mototani Y, Ishigaki S, Kon Y, Kasai N (2003). "Bilateral congenital cataracts result from a gain-offunction mutation in the gene for aquaporin-0 in mice". Genomics 81 (4): 361–8. doi:10.1016/S0888-7543(03)00029-6. PMID 12676560. 33) ^ Khanna A (2006). "Acquired nephrogenic diabetes insipidus". Semin. Nephrol. 26 (3): 244–8. doi:10.1016/j.semnephrol.2006.03.004. PMID 16713497. 34) ^ Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR (2005). "IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 transport channel". J. Exp. Med. 202 (4): 473–7. doi:10.1084/jem.20050304. PMC 2212860. PMID 16087714. 35) HELIX 1J4N 36) HELIX 37) LoopA 38) HELIX 39) ----40) HELIX 41) ----42) HELIX 43) LoopB 44) HELIX 45) HELIX 46) LoopC 47) HELIX 48) ----49) HELIX 50) LoopD 51) HELIX 52) LoopE 53) HELIX 54) HELIX 55) REMARK 56) REMARK 57) REMARK 58) REMARK 59) REMARK 60) REMARK 61) REMARK 62) REMARK 63) REMARK 64) REMARK 65) REMARK 66) REMARK 67) REMARK 68) REMARK 69) REMARK 70) REMARK 71) REMARK 72) REMARK 73) REMARK 74) REMARK 75) REMARK 76) REMARK 77) REMARK 78) REMARK 79) REMARK 80) REMARK 81) REMARK 82) REMARK 83) 1 1 PHE H1 2 2 PHE 3 4 5 6 7 8 9 10 11 12 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 465 5 GLY A 34 1 35 TYR A 37 5 38 49 3 ASP H2 50 GLY A 74 1 75 77 4 ASN A 78 SER A 88 1 89 91 5 SER H3 92 THR A 118 1 119 137 6 GLY A 138 GLY A 140 5 7 LEU H4 141 THR A 159 1 160 168 8 SER H5 169 GLY A 190 1 191 193 9 ASN A 194 THR A 205 1 206 211 10 TRP H6 212 PHE A 231 1 232 238 11 ASP A 239 LYS A 245 1 12 VAL A 246 THR A 248 5 MISSING RESIDUES 1J4N THE FOLLOWING RESIDUES WERE NOT LOCATED IN THE EXPERIMENT. (M=MODEL NUMBER; RES=RESIDUE NAME; C=CHAIN IDENTIFIER; SSSEQ=SEQUENCE NUMBER; I=INSERTION CODE.) M RES GLY GLN VAL GLU GLU TYR ASP LEU ASP ALA ASP ASP ILE ASN SER ARG VAL GLU MET LYS PRO LYS A C SSSEQI A 250 GLY A A 251 GLN A A 252 VAL A A 253 GLU A A 254 GLU A A 255 A 256 A 257 A 258 A 259 A 260 A 261 A 262 A 263 A 264 A 265 A 266 A 267 A 268 A 269 A 270 A 271 1FQY 248 249 250 251 252 5 30 3 25 11 27 3 19 22 12 20 7 3