porous carbon Structure from Rice bran for double-layer
advertisement

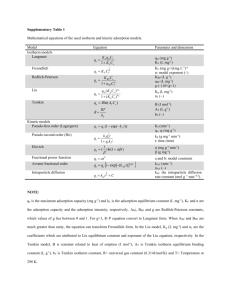
Supporting Information for From Rice Bran to High Energy Density Supercapacitors: A New Route to Control Porous Structure of 3D Carbon Jianhua Hou, a Chuanbao Cao, a* Xilan Ma, a Faryal Idrees, a Bin Xu, b Xin Hao b and Wei Linb a Center of Materials Science, Beijing Institute of Technology, Beijing 100081, P. R. China b State Key Laboratory of Chemical Resource Engineering, Beijing Key Laboratory of Electrochemical Process and Technology for Materials, Beijing University of Chemical Technology, Beijing 100029, P. R. China. Corresponding Author: E-mail address: cbcao@bit.edu.cn Tel: +86 10 6891 3792; Fax: +86 10 6891 3792; Figure S1. The typical of RBC-X electrode’s Radius and height (Formula s1 is used to calculate density of RBC-X electrode). ρ m m m 2 v s h πr h (S1) By using S1 formula density of electrode material calculated are: 0.45 g cm-3 for RBC-4, 0.46 g cm-3 for RBC-3, 0.56 g cm-3 for RBC-2 and 0.79 g cm-3 for RBC-1. Table S1. Combustion elemental analysis and XPS result for the RB and RBCs. Elemental analysis (wt.%) Sample C N H S RB 39.72 6.64 2.13 RBC 77.81 4.65 RBC-1 84.32 RBC-2 XPS (wt.%) C O N Cl 0.43 45.34 47.92 6.74 0.00 1.68 0.34 82.28 12.77 4.83 0.12 1.99 1.53 0.36 88.15 10.28 1.57 0.00 87.45 1.03 1.27 0.28 90.07 8.77 1.12 0.04 RBC-3 91.67 0.74 1.06 0.15 92.56 6.55 0.78 0.11 RBC-4 92.57 0.41 1.02 0.21 95.12 4.33 0.52 0.03 YP-17D 92.43 0.15 1.43 0.00 - - - - C 60000 50000 40000 30000 intensity (a.u.) 70000 N O RBC RBC-1 RBC-2 RBC-3 RBC-4 20000 10000 0 0 200 400 600 800 Binding energy (eV) Binding energy (eV) Figure S2. XPS survey spectrum of the RBCs. 1000 a -1 b 1.0 IR RBC-4 RBC-3 RBC-2 RBC-1 200 Voltage (V) Specific Capacitance(F g ) 300 100 0 10 mV s -1 -100 -200 0.0 0.2 0.4 0.6 Voltage (V) 0.8 0.6 0.4 0.2 RBC-3 RBC-1 RBC-4 RBC-2 -300 0.8 0.0 1.0 0 50 100 150 Time (s) 200 250 Figure S3. Electrochemical performance characteristics of RBC-X measured in a two-electrode system in the 6 M KOH electrolyte. (a) CV curves at the scan rate of 10 mV s−1; (b) Galvanostatic charge–discharge curves at current density of 1 A g−1 b a -1 2Ag -1 5Ag -1 10Ag -1 20Ag -1 30Ag -1 40Ag -1 50Ag 1.0 200 -1 5mVs -1 20mVs -1 50mVs -1 200mVs -1 500mVs 100 0 -100 Potential(V) -1 Specific Capacitance(Fg ) 300 -200 0.8 0.6 0.4 0.2 -300 0.0 0.2 0.4 0.6 Potential(V) 0.8 1.0 0.0 0 20 40 60 80 Time (s) 100 120 140 Figure S4. (a) Cyclic voltammograms of RBC-4 at various scan rates; (b) Galvanostatic charge–discharge curves of RBC-4 at different current densities. 60 15 40 -z"(ohm) -z"(ohm) 50 30 5 20 10 0 0 10 0 10 0 20 5 10 z'(ohm) 30 40 z'(ohm) 50 15 60 Figure S5. Nyquist plots and its expanded high frequency region (inset). Table S2 Comparison of specific capacitance for the obtained activated carbon sample with those of previously reported samples. Precursor rice husk SBET (m2 g-1) 1565 sunflower seed shell 2509 animal bone 2157 glucose bacillus subtilis 2096 1578 Capacitance (F g-1) 245a 0.05 A g-1 233a 2 A g-1 311 a 0.25 A g-1 a microporous carbon nanosheets mesoporous carbon thin films EM-CCG film Carbon nanocages silk rice bran 2264 791 690 557167c 1854 2557 2475 -1 144 185a 10 A g 0.05 A g-1 143a 10 A g-1 221a 2 mV s-1 141a 200 mV s-1 310b 0.2 A g-1 200b 10A g-1 190a Ph-POSS Current density Electrolyte Ref. 6 M KOH S1 30.% KOH S2 7 M KOH S3 1 M H2SO4 S4 6 M KOH S5 6 M KOH S6 0.02 A g-1 110a 2 A g-1 213b 0.5 A g-1 160b 10 Ag-1 136b 0.5 A g-1 85b 5 A g-1 192a 0.1 A g-1 171a 1 A g-1 146a 10 A g-1 260a 0.1 A g-1 178a 10 A g-1 112a 100 A g-1 264a 0.1 A g-1 162 a 6.2 A g-1 120 a 52.5 A g-1 323a 0.1 A g-1 265a 10 A g-1 182a 100 A g-1 6 M KOH S7 6 M KOH S8 1 M H2SO4 S9 1 M H2SO4 S10 2 M H2SO4 S11 6 M KOH This work a A two-electrode system was used. b A three-electrode system was used. c The SSA values obtained from the methylene blue (MB) adsorption are only valid for macro- and mesoporous materials9. S1. He, X. et al. Rice husk-derived porous carbons with high capacitance by ZnCl< sub> 2</sub> activation for supercapacitors. Electrochimica Acta 105, 635-641 (2013). S2. Li, X. et al. Preparation of capacitor’s electrode from sunflower seed shell. Bioresour. Technol. 102, 1118-1123 (2011). S3. Huang, W.T. Zhang, H. Huang, Y.Q. Wang, W.K. & Wei, S.C. Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors. Carbon 49, 838-843 (2011). S4. Estevez, L. et al. A facile approach for the synthesis of monolithic hierarchical porous carbons–high performance materials for amine based CO2 capture and supercapacitor electrode. Energy Environ. Sci. 6, 1785-1790 (2013). S5. Zhu, H. Yin, J. Wang, X. Wang, H. & Yang, X. Microorganism-derived heteroatom-doped carbon materials for oxygen reduction and supercapacitors. Adv. Funct. Mater. 23, 1305-1321 (2013). S6. Li, Z et al. Synthesis of well defined microporous carbons by molecular scale templating with POSS moieties. J. Am. Chem. Soc. 136, 4805-4808 (2014). S7. Jin, Z.Y. Lu, A.H. Xu, Y.Y. Zhang, J.T. & Li, W.C. Ionic liquid-assisted synthesis of microporous carbon nanosheets for use in high rate and long cycle life supercapacitors. Adv. Mater. 26, 3700-(2014). S8. Dan, F. et al. Free-standing mesoporous carbon thin films with highly ordered pore architectures for nanodevices. J. Am. Chem. Soc. 133, 15148-15156 (2011). S9. Yang, X. Cheng, C. Wang, Y. Qiu, L. & Li, D. Liquid-mediated dense integration of graphene materials for compact capacitive energy storage. Science 341, 534-537 (2013). S10. Xie, K. et al. Carbon Nanocages as Supercapacitor Electrode Materials. Adv. Mater. 24 347-351 (2012). S11. Yun, Y. S. et al. Microporous carbon nanoplates from regenerated silk proteins for supercapacitors. Adv. Mater. 25 1993-1998 (2013).