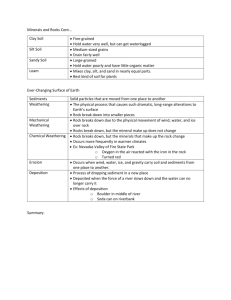
Soils from andesites
advertisement

Soils: chemical transformations during weathering and soil formation Stephen U. Aja Weathering Weathering is the process of breakdown of rocks at the surface of the earth and reflects an adjustment of rocks to conditions of temperature, humidity and fluid composition on the earth’s surface. Weathering processes may be classified into two main types, namely physical (or mechanical) and chemical weathering. During physical weathering, rocks break in response to stresses that have been established within the rock. The breaking may occur along fracture planes in the rock or along mineral grain boundaries. Physical weathering does not produce any change in the chemical composition of the rock rather the rock becomes broken up into fragments smaller than the initial volume of rock. Chemical weathering, on the other hand, are chemical changes undergone by rocks exposed near the earth’s surface. Rocks typically form in pressure and temperature conditions far removed from those at or near the earth’s surface. Hence, when such rocks become exposed to the water compositions, temperature and pressure conditions on the earth’s surface, the rocks will adjust themselves to the prevailing surficial geochemical conditions. This adjustment produces significant chemical changes in the composition of the rock such that the major element chemistry of the weathered material is distinct from that of the parent rock. These chemical changes are manifested by depletion of the original minerals in the rock, formation of clay minerals, changes in the chemistry of water draining the rock and also changes in the color of iron-bearing minerals. The agents of chemical weathering include water, carbon dioxide, oxygen and organic acids; organic acids are derived from the alteration of plant litter. Water provides the medium for dissolution of minerals and the breakdown of complex minerals such as feldspars by reaction with water is known as hydrolysis reactions. The effect of hydrolysis reactions is to make an aqueous solution more basic. Carbon dioxide makes rainwater moderately acidic; this increased acidity leads to increased dissolution of minerals during weathering. Oxygen is an important agent for the weathering of minerals containing iron. In iron-bearing rock-forming minerals(e.g., olivine, pyroxene), iron occurs in a reduced state whereas in weathering or near-surface environments, iron occurs in the oxidized state as in minerals such as hematite (Fe2O3) and limonite (Lab 1). Organic acids, released by decomposing plants, increase the rate of rock weathering by increasing the rate at which metals such as Fe or Al are removed during weathering. 1 Chemical weathering reactions of iron minerals As noted previously, pyroxene is an iron-bearing mineral found in igneous rocks such as basalt. Equation 1 represents a weathering reaction of the pyroxene to form hematite. 4 iron pyroxene + oxygen + 8 water → 2 hematite + 4 dissolved silica (1) 4 FeSiO3 ( s ) O2 ( g ) 8 H 2O( l ) 2 Fe2O3 ( s ) 4 H 4 SiO4 ( aq) In the various equations employed in this exercise, the physical states of the different species in the reactions are given by the following subscripts s, l, aq and g; these symbols indicate that the species are in solid, liquid, aqueous and gaseous states, respectively. Also in equation 1, stoichiometric coefficients are the numbers that appear before each reacting species such as the 4 before iron pyroxene. The stoichiometric coefficient is a measure of the reacting units and these reacting units represent the number of moles of each species involved in the reaction. Problem 1. In the chart below, identify the reactants and products in equation 1, their physical states, and number of moles (or stoichiometric coefficient) of each species. Identification of species in equation 1 Reactant Physical Stoichiometric Product Species State coefficient Species Physical State Stoichiometric coefficient Problem 2. A homogenous reaction is one in which all the reacting species are in the same state (gas or liquid or solid) whereas a heterogeneous reaction has reacting species in mixed states. Is reaction 1 a heterogeneous or homogenous reaction? During weathering, a mineral may be dissolved completely or may leave a residue. If the dissolution is complete, as when a mineral such as halite (table salt) is added to water, the dissolution is known as congruent. But if the dissolution is partial and thus leaves a residue (or precipitate), the dissolution is known as an incongruent dissolution. Problem 3. Does reaction 1 represent a congruent or an incongruent dissolution? Refer to the definition of congruent and incongruent dissolution in the discussion above. 2 Chemical Budget Problem 4. According to reaction 1, four (4) moles of pyroxene weathered to two (2) moles of hematite. How many moles of hematite will the weathering of half a mole (0.5 moles) of pyroxene produce? Oxidation states of Fe Problem 5. During the weathering of iron minerals, the Fe undergoes a change in oxidation state (or the charge carried by the iron). In equation 1, the weathering of iron pyroxene (FeSiO3) produces hematite (Fe2O3). [The net charge on minerals is zero; that is the number of positive charges must equal the number of negative charges. In minerals, the charge of Si is usually +4 and that of O is usually -2]. Calculation of the charge of Fe in pyroxene and hematite Hints Pyroxene (a) Inspect mineral formula No of oxygen atoms in given above mineral (b) Multiply number of Total negative charge oxygen atoms by –2 from oxygen (c) Inspect mineral formula No of silicon atoms in given above mineral (d) Multiply number of Total positive charge silicon atoms by +4 from silicon (e) Find the sum of the Unsatisfied negative positive and negative charge charges (f) Must be equal to the Positive charges needed unsatisfied negative to balance negative charges charges (g) Inspect the formula of the Number of Fe atoms in mineral mineral Divide “f” by “g” Oxidation state of Fe Hematite From your calculation in the chart above, does the charge of Fe (oxidation state of Fe) increase or decrease during the weathering reaction shown in equation 1? ______________________________________________________________ Circle the correct answer: the charge of Fe in primary minerals increases or decreases with weathering. 3 Weathering reactions of feldspar Plagioclase feldspar is one of the feldspars examined in Lab 1 (on minerals); the other being orthoclase feldspars. The weathering of feldspars may be described as a hydrolysis reaction where hydrolysis is the reaction of silicate minerals with water. Some aspects of these hydrolysis reactions are shown in reactions 2, 3 and 4 (below). Plagioclase feldspar + 4 water + 4 hydrogen ion → sodium ion + aluminum ion + 3 silicic acid NaAlSi3O8( s ) 4 H 2O( l ) 4 H (aq) (2) 3 Na(aq) Alaq 3H 4 SiO4 ( aq) Problem 6. Does equation 2 represent a congruent or incongruent dissolution? Problem 7. Based on equation 2, do you expect the hydrogen ion concentration ( H aq ) in water reacted with plagioclase to increase or decrease? Explain your answer. Meaning of pH pH is a measure of the acidity/basicity of a solution and acidity is determined by the concentration of hydrogen ions in the solution. The acidity of a solution is referenced to the pH scale which varies from 0 to 14; acidic and basic (alkaline) solutions have pH ranges of 0 to 7 and 7 to 14, respectively. pH is defined as the negative logarithm of the hydrogen ion concentration or pH log 10 ( H ) ; therefore, (H+) = 10-pH. Problem 8. The typical range of pH for minerals soils in humid and arid regions are 5 to 7.5 and 6.5 to 9, respectively. Given two soil solutions whose pH values are 7.5 and 8.5, by how much does the hydrogen ion concentrations of the two soils differ differ? 4 Problem 9. Do you expect the pH of the solution reacted with feldspars (such as equation 2) to increase or decrease with time (or reaction progress)? Why? Plagioclase + 4.5 water + hydrogen ion → 0.5 kaolinite + 2 silicic acid + sodium ion (3) NaAlSi3O8 ( s ) 4.5 H 2O( l ) H (aq) 0.5 Al2 Si2O5 (OH ) 4( s ) 2 H 4 SiO4 Na(aq) Problem 10. Which of the above reactions, 2 or 3, would you expect to occur in a more acidic environment such as a region affected by acid rain? Explain your answer. Plagioclase feldspar weathering in the presence of CO2 Normally, rainwater dissolves some carbon dioxide as it passes through the atmospheric column. If the water reacting with the plagioclase feldspar is saturated with carbon dioxide from the atmosphere, the reaction may then be written as: Plagioclase + 4.5 water + carbonic acid → 0.5 kaolinite + sodium ion + bicarbonate ion + 2 silicic acid (4) NaAlSi3O8( s ) 4.5H 2O( l ) H 2CO3( aq) 0.5 Al2 Si2O5 (OH ) 4( s ) Naaq HCO3( aq) 2H 4 SiO4( aq) Problem 11. Are there any differences in either the type or amounts of the solid products formed in reactions 3 and 4? What are the differences, if any? Problem 12. Which of the two weathering models, reaction 3 or 4, will occur at a slower rate? Why? [Hint: assume that the active ingredient driving the reaction is the concentration of hydrogen ions.] 5 Problem 13. What determines the amount of solid products formed at the end of the weathering event, the rate of reaction (how quickly the reaction proceeds) or the amount of fresh rocks weathered? Problem 14. Considering your answers to questions 10 and 11, under which weathering condition, reaction 3 or 4, is the formation of a well-developed soil from the parent rock likely to require the least amount of time? Explain your answer. Soil Formation processes and factors Soil, the thin veneer on the earth’s land surface, consists of mineral matter (45%), organic matter (5%), pore spaces filled with air (25%) and water (25%). Soils are the residual products of weathering; the intensity of weathering processes decreases from the exposed surface of the bedrock downwards. This means that the part of the bedrock exposed to weather (i.e., rain, snow, ice, heat) will undergo the most alteration. Soil thicknesses may vary from 0.3 to 2m or more. Because alteration proceeds from the exposed surface downwards, all soils are vertically zoned beginning with a humus-rich layer (or layer rich in altered plant matter) at the surface to the least altered mineral layer just above the unweathered parent rock. Figure 1: Idealized soil profile A well-developed soil is one whose constituent horizons (or soil profile) are clearly resolved (see Figure 1). The major processes responsible for the development of soil profiles include additions (atmospheric precipitations and organic matter as plant tissue), 6 transformations (of minerals and organic matter), translocations (of clays, humus, aqueous ions from one level in the soil to the other) and leaching or removals (of soluble ions from weathered layers). The factors that influence these processes of soil formation include the type of parent rock (bedrock), the local topography (steep or gentle slope), the type of climate (humid, dry, warm, cold), and the amount of time that has elapsed. Climate parameters such as humidity and temperature are the overwhelming factors in soil formation. Temperature increases the rates weathering reactions and water (from rainfall) provides the medium in which chemical weathering reactions can occur. Chemical weathering reactions will not occur in hot, dry environments. Different rocks contain different minerals and these minerals weather at different rates under the surface condition of the earth. The less stable minerals (easily altered) at earth’s surface conditions will undergo a greater extent of weathering. Secondly, the type of soil produced by weathering also depends on the weathered bedrock. For instance, a rock that is rich in both feldspars and quartz is likely to produce a sandy clayey soil whereas a parent rock that lacks free quartz (e.g., basalt) is unlikely to produce any type of sandy soil. The topography of the weathered area also influences soil formation; a rugged and steep slope will work against deep chemical weathering and therefore poor soil development. Problem 15. In which horizon of the soil profile (Figure 1), would you expect to find the greatest concentration of clay minerals (such as kaolinite)? Explain your answer. Problem 16. Organic acids released by the decay of plant matter enhance weathering under forested areas. Usually, the presence of organic acids enhances the leaching reaction of iron and aluminum. In the soil profile, the A horizon is the only layer that may contain both organic and mineral matter. If organic acids played important roles in the weathering processes, where in the soil profile would you expect its leaching effect to be most pronounced? Between O and A horizon Between A and B horizon Between B and C horizon ___________________ ___________________ ___________________ Explain your answer 7 Weathering of Pliocene andesites: a quantitative treatment The mineral composition of two Pliocene andesites from the Cascade Range of NE California (Hendricks and Whittig, 1968; Journal of Soil Science, volume 19, pp 135146, 147-153) is depicted in Figure 2. Problem 17. From fig. 2, list the minerals in the andesites in the order of decreasing abundance? Hypersthene andesite: Olivine andesite: How do the mineral compositions of the two rocks differ? Hypersthene Andesite plagioclase phenocrysts plagioclase microlites hypersthene olivine mafic groundmass opaques glass Olivine Andesite plagioclase phenocrysts plagioclase microlites hypersthene olivine mafic groundmass opaques glass Figure 2: Mineralogy of the unaltered andesites: A) hypersthene andesite and B) olivine andesite. Microlites are primarily very fine-grained crystals of plagioclase (that may also contain quartz and potash feldspar because of their fine grain sizes) whereas the phenocrysts are very large crystals of plagioclase. The composition of the hypersthene andesite is phenocrysts (21%), microlites (47%), hypersthene (21%), opaques (3%), glass (8%) whereas the olivine andesite consists of phenocrysts (13%), microlites (47%), hypersthene (6%), olivine (8%), mafic (Mg and Fe –rich) groundmass (20%), opaques (4%) and glass (2%). Exploded slice shows glass composition. 8 Soils from andesites The soils formed from the olivine and hypersthene andesites are formally described as Cumulic Ustic Umbrihumult (soil order, Ultisol) and Andic Haplumbrept (soil order, Inceptisol), respectively (Hendricks and Whittig, 1968). The soil formed from the olivine andesite is thus a thick, dark colored soil having a high humus content, low base (Ca2+, Mg2+, K+) saturation and strong structure. Its soil moisture content (ustic) is intermediate between soils having year-round plant-available moisture and those with water supply for about half of the plant-growing season; its subsurface horizons (cumulic) contain clay accumulations. The olivine andesite weathered to a fine, loamy soil with a profile thickness of 120 cm. The soil formed from the hypersthene andesite has dark-colored surface horizons with medium-to-low basic cation supply. It is characterized by high content of volcanic glass, amorphous materials or poorly crystalline iron and aluminum oxides and oxyhydroxides. This soil has a minimum horizon development (“Hapla”) and is characterized by the presence of amorphous (short-range order) materials formed from the weathering of volcanic glass (“andic”). The hypersthene andesite weathered to a coarse loamy soil with a profile thickness of 118 cm. A loam is a soil having moderate amounts of sand, silt and clay and a loamy soil has properties intermediate between a fine-textured (“ashy”) and coarse-textured (“silty”) soil. Problem 18. The hypersthene and olivine andesites have a combined iron oxide compositions of 6.7 % and 8.1%, respectively. Which of these two andesites is likely to produce a more intense reddish-colored soil? Problem 19. How would you explain the fact that the hypersthene andesite formed a coarse loamy soil whereas the olivine andesite produced a fine loamy soil? [Hint: consider the mineral composition of the andesites shown in Fig. 2]. Mineralogy of Saprolites The mantle of unconsolidated material that lies on solid unweathered rock is known as regolith (and includes soil) whereas the term saprolite refers to chemically weathered rock that that retains the structure of the original rock and lies below the soil; usually saprolite has not experienced any transportation. 9 There are 4 stages of saprolite alteration of the andesites in figure 2 but only the initial and final stages of alteration are shown in Table 1. The partial mineral compositions of the stage 4 saprolites are shown below. Table 1. Approximate mineral compositions of stage 4 saprolites Percent Composition (%) Stage 4 saprolite olivine andesite Quartz Feldspar (orthoclase and plagioclase) Mica-like clay Amorphous phases (non-crystalline solids) Kaolinite Iron oxide 1 <1 2 27 30 10 hypersthene andesite 2 <1 2 28 38 8 Problem 20. Using the information in table 1 (stage 4 saprolites) and figure 2, list the minerals or other solids present in greatest abundances in the chart below. Hypersthene andesite (figure 2) Stage 4 saprolite (from table 1) Olivine andesite (figure 2) Stage 4 saprolite (from table 1) From your analyses above, are the minerals present in greatest abundance in the saprolites the same as in the andesites? ________________________________________________________________ If not, list the minerals present in greatest abundances in the andesites and the ones present in greatest abundances in the saprolites. Andesites: Saprolites: Problem 21. Can the mineral abundances in the saprolites and in the andesites be explained using feldspar hydrolysis reactions (i.e., equations 2 to 4 above)? Explain. 10 Abrasion pH Abrasion pH is the pH measured in aqueous solutions produced by grinding rocks or minerals in distilled water; abrasion pH for the hypersthene andesite, olivine andesite, and the corresponding values for stage and 1 and 4 saprolites are shown in the chart below. Solid material Hypersthene andesite Abrasion pH 8.9 Solid material Olivine andesite Abrasion pH 8.6 Stage 1 saprolite 5.5 Stage 1 saprolite 6.3 Stage 4 saprolite 4.9 Stage 4 saprolite 5.4 Problem 22. Compare the abrasion pH of the saprolites and unweathered andesites. How does the abrasion pH vary with weathering: increase, decrease or stay the same? Problem 23. What is the concentration of hydrogen ions in the stage 4 saprolite? By how much does the hydrogen ion concentration in the fresh rock and saprolite waters differ? Problem 24. Since aqueous solutions generally increase in pH as the solids undergo hydrolysis, could the trend of abrasion pH be attributed to hydrolysis? Problem 25. If the abrasion pH were not due to hydrolysis, what would be an alternative explanation? [Hint: consider whether hydrogen ions are being consumed or release back into the solution and also the types of minerals present in the saprolite and in the fresh rock.] 11 Extent of alteration of fresh rocks Problem 26. For the olivine andesite, approximately 22, 43, 44 and 45 % of original rock is removed at each of the 4 stages of saprolite alteration. During which phase of alteration was the most rock matter removed and why? Problem 27. During the saprolite formation, 47.4 % and 45.4 % of the original hypersthene and olivine andesites, respectively, were lost. If there were 1 kg of original rock present, how much rock would be left over at the end of each stage of weathering? Hypersthene andesite: Olivine andesite: Problem 28. Which of the two rocks (hypersthene andesite or olivine andesite) experienced a faster rate of weathering? For the andesitic rock that weathered at a faster rate, was its rate of weathering moderately or considerably greater? On what observations are your inferences based? 12 13