plasma magnesium and cardiovascular changes
advertisement

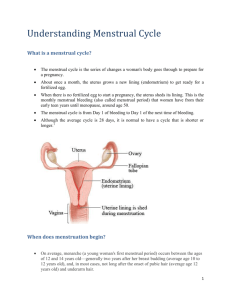
PLASMA MAGNESIUM AND CARDIOVASCULAR CHANGES DURING MENSTRUAL CYCLE IN YOUNG NIGERIAN WOMEN 1Olayaki L.A., Salman T.M., Ayinla M.T., Soladoye A.O. and 2M.S. Ajao 1Department of Physiology, 2Department of Anatomy, Faculty of Basic Medical Sciences, College of Health Sciences, University of Ilorin, P.M.B. 1515, Ilorin *Correspondence: Luqman A. Olayaki Department of Physiology and Biochemistry, Faculty of Basic Medical Sciences, College of Health Sciences, University of Ilorin, P.M.B. 1515, Ilorin, Kwara State, Nigeria. Tel.:234-8033814880; e-mail:luqmanolayaki@yahoo.com ABSTRACT This study was designed to determine the pattern and magnitude of changes in plasma magnesium (Mg2+) in relation to Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), and Heart Rate (HR) during the menstrual cycle. Plasma Mg2+ was measured in fasting blood samples collected in the morning three times spread over the three phases of the menstrual cycle: Early Follicular (EF: days 1-3, menstruation), Periovulatory (PO; days 14-15 of the cycle), and mid-luteal (ML; days 21-23 of the cycle) respectively, in twenty healthy women aged 19-24 years and with regular menstrual cycle (27-30 days). SBP, DBP, and HR were also determined. The results obtained showed plasma Mg2+ decreased from 1.85±0.30 mg/dl during early follicular phase to 1.43±0.37 mg/dl during the periovulatory phase (p<0.05), and then increased to 1.76±0.32mg/dl during the mid-luteal phase (p<0.05). SBP increased from 114.35±4.87 mmHg during the early follicular phase to 119.20±4.70 mmHg during the periovulatory phase (p<0.05), and then fell to 114.10±5.48 mg/dl during the midluteal phase (p<0.05). The DBP increased from 64.45±4.05 mmHg during the early follicular phase to 68.05±3.41 mmHg during the periovulatory phase (p<0.05) and then decreased to 64.90±3.09 mmHg during the mid-luteal phase (p<0.05). The HR increased from 69.30±7.24 beats/minute during the early follicular phase to peak at periovulatory phase 73.60±7.65 beats/minute. It then fell to 70.35±7.20 beats/minute during the midluteal phase. Though there is no significant difference in HR across the phases of the menstrual cycle. 1 In conclusion, these results indicate that blood Mg2+ levels fluctuate during the menstrual cycle along with SPB and DPB and could be one of the factors responsible for changes in cardiovascular parameters during the menstrual cycle. Key words: Plasma Magnesium, Systolic Blood Pressure, Diastolic Blood Pressure, Menstrual Cycle. 2 INTRODUCTION Recent studies demonstrated that menstrual cycle is associated with variation in haemodynamics1,2,3, sympathetic and immune activities4. Dietary Mg2+ has been inversely associated with blood pressure in populations5,6,7,8, variations in BP9,10, and HR 11,10 . Variations in blood pressure have been reported across the menstrual cycle, and Mg2+ deficiency has been linked to the pathogenesis of hypertension12. Mg2+ therapy has been shown to be useful in the treatment of cardiac dysrythmias13, acute myocardial infarction14, acute cerebral ischemia15,16, pre-eclampsia and eclampsia17,18. Mg2+ salts have been shown to lower BP19. MARERIALS AND METHOD Twenty young women, who were students of University of Ilorin, participated in the study. They were aged between 19-24 years with menstrual cycles that were regular and 27-30 days duration. Inclusion criteria for the study were the following: 1) maintaining regular menstrual cycles of 27-30 days, with the cycle duration not changing by more than two days for the three prior months, 2) not taking any type of prescribed medication including oral contraceptives for at least one year prior to study, 3) maintaining a body weight with a desirable body mass index (18 – 25 kg/m2), 4) not taking alcohol, 5) nonsmoking and non-dieting, 6)no past or ongoing chronic illness, 7) not pregnant or lactating for one year prior to the study and 8) not exercising for more than 60 minutes a day or seven hours a week. The subjects were also certified medically fit by the 3 university clinic physician. They had no breakfast and abstained from strenuous exercise before study. Informed consent was obtained from each subject. Height and weight were recorded, and body mass index (BMI, kg/m2), which is the body weight (in kg) divided by the square of the height (in m), was calculated. Height was measured without shoes using a standiometer that is a non-stretchable tape attached to a vertical flat surface (wall), with a right-angle headboard. A beam scale with nondetachable weight was used to measure weight with clothes but no shoes. During each visit, a total of 5mls of blood was obtained by venepuncture and collected into lithium heparinised tubes. Blood collections for each subject were performed at approximately the same time of the day throughout the study period to reduce variability within each individual. Immediately after the collection, plasma and erythrocytes were separated by centrifugation. Centrifuged samples were stored at -40C, until analysis was done. Plasma Mg2+ was determined by colometric method using xylidyl blue reagent and glycohetherdiamine tetraacetic acid (GEDTA) as diluting agent. Table 1: See page 13. Statistical Analysis Results are expressed as mean ± SEM, and n denotes the number of subjects. The values were assessed for statistical significance by one-way analysis of variance (ANOVA). A probability level of p<0.05 was considered significant. RESULTS Table 2: See page 13. 4 Changes in plasma Mg2+ In table 2, mean ± SEM of plasma Mg2+, in the three phases of menstrual cycle and corresponding p-values are presented. Periovulatory phase was associated with lowest Mg2+ 0.80±0.05 mmol/L. In the early follicular phase, the Mg2+ was 1.10±0.11 mmol/L, and decreased to 0.80±0.05 mmol/L in the periovulatory phase (p<0.001), (fig.4). The Mg2+ was increased to 0.98 ± 0.08 mmol/L in the mid-luteal phase (p<0.05), (fig.4). Changes in blood pressure and Heart Rate Table 2 shows the mean ± SEM values of SBP and DBP in the three phases of menstrual cycle. SBP increased from 115.15±5.73 mmHg in the early follicular phase to 119.0±4.70 mmHg (p<0.05, n=20), (fig.1) in the periovulatory phase and reduced to 114.10±5.48 mmHg in the mid-luteal phase (p<0.05, n=20), (fig.1) DBP increased from 68.50±3.73 mmHg in the early follicular phase to 71.50±4.42 mmHg in the periovulatory phase (p<0.05, n=20), (fig.2), and reduced to 66.50±3.65 mmHg in the mid-luteal phase. However, HR was similar in the phases of the three phases of the menstrual cycle, (fig.3). DISCUSSION The results of this study demonstrated that plasma Mg2+ show distinct variations during menstrual cycle in healthy young women. Plasma Mg2+ decreased significantly from a lower level during EF phase to a maximum level during the PO phase, which coincides with the lowest SBP and DBP. Thereafter, the plasma Mg2+ increased to a higher level during the ML phase during which SBP and DBP were at their lowest level. Although variations in plasma Mg2+ concentration were observed across the menstrual cycle, the 5 values at each phase were always within the reference range for healthy adults (0.8-1.3 mmol/L). In most studies negative relationships between urinary Mg2+ excretion and blood pressure have been established20. Mg2+ is important in activating the Na+-K+ ATPase pump which in turn, expedites the movement of potassium into the cell and sodium out of the cell21. Mg2+ is also important in decreasing the entry of Ca2+ into the cell12; hence Mg2+ deficiency leads to increased intracellular sodium and Ca2+, increased peripheral resistance and vasospasm. By regulating smooth muscle tone, Mg2+ may play a role in illnesses such as acute myocardial infarction, hypertension, acute cerebral ischemia, and asthma exacerbation. Smooth muscle tone is determined by Ca2+ dependent phosphorylation of myosin light chain22. Higher levels of intracellular Ca2+ are associated with more smooth muscle constriction. Mg2+ regulates the intracellular Ca2+ levels and thereby influences smooth muscle tone22. Mg2+ deficiency is associated with an increase in intracellular Ca2+ and increased smooth muscle vasoconstriction. Mg2+ salts have been shown to lower blood pressure19, and it is a naturally occurring antagonist of Ca2+ 23. Mg2+ has also been shown to affect cardiac contraction, beating rhythm, vasomotor control, and proliferation of smooth muscle cells in vessels24. The mechanisms for the observed cyclic changes in Mg2+ parameters during the menstrual cycle are uncertain. Estradiol may be a physiological modulator, regulating Mg2+ concentration directly or indirectly. It has been suggested that oestrogen plays a protective role against cardiovascular disease, partly by decreasing the likelihood of low- 6 density lipoprotein perioxidation25,26. Our results suggest that effects of oestrogen may also be responsible for modulation of CVS parameters. In the present study, the increased in SBP observed during the PF coincided with a decrease in the plasma Mg2+during the PF. It is possible that the change in plasma Mg2+ could be responsible for the increase in SBP and DBP. Oestrogen is known to enhance Mg2+ utilization and uptake by soft tissues and bones27, and this may be one of the mechanisms by which oestrogen contribute to resistance of young women to heart diseases, as well as increased prevalence of these diseases when oestrogen secretion ceases. It has been observed that patterns of 17-βestradiol concentration observed over four points during menstrual cycle fluctuated significantly over the menstrual cycle with lowest concentration during the early follicular phase and highest during the periovulatory phase and decreased by about 50% during the mid-luteal phase28. Mg2+ might even have a stronger effect on the cyclic nature of cardiovascular parameters during the menstrual cycle. It could be seen that the lowest concentration of plasma Mg2+ is associated with the highest levels of systolic and diastolic blood pressure. It was at the period of expected highest plasma oestrogen concentration. These results indicate that the phase of the menstrual cycle should be considered when Mg2+ status is assessed in women of reproductive age. In the current study, plasma Mg2+ concentrations decreased by approximately 9% during the periovulatory phase. Marginal Mg2+ status therefore, could be misinterpreted as inadequate if blood sampling occurred during this phase. 7 The results of this study also suggested that plasma Mg2+ characteristics and fluctuations should be considered when establishing cardiovascular parameters in premenopausal women. It is likely that a decreased in plasma Mg2+ may be accompanied by an increased in systolic and diastolic blood pressure. Because of the significant increase in SBP and DBP during the periovulatory phase when there was highest concentration of plasma oestrogen and lowest concentration of plasma Mg2+, plasma Mg2+ might be one of the factors influencing the cyclical changes in blood pressure across the phases of menstrual cycle in young women. 8 REFERENCES 1. Hall VL, Leathard HL. Menstrual cycle influences on cardiovascular functioning. J Physiol 1999; 515:78-79. 2. Spaanderman MEA, van Beck E, van Eyck ET. Changes in haemodynamic parameters and volume haemostasis with the menstrual cycle among women with a history of pre-eclampsia. Am J Obstet Gyneacol. 2000; 182:1127-1134. 3. Soladoye AO. Haemorrheological Changes During Menstrual Cycle Among Nigerians Women. Tropical J Health Sci 1995; 2:17-20. 4. Shakhar K, Shakar G, Rosenne E. Timing within the menstrual cycle, sex, and the use of oral contraceptives determine adrenergic suppression of NK cell activity. Br J Cancer 2000; 83:1630-1636. 5. Reed D, McGee D, Yann K, Hankin J. Diet, Blood Pressure and Multicollinearity. Hypertension. 1985; 7:405-410. 6. Joffres MR, Reed DM, Yano K. Relationship of magnesium intake and other dietary factors to blood pressure: The Honolulu heart study. Am J. Clin. Nutri. 1987; 45:469-475. 7. Kesteloot H, Joossens JV., Relationship of dietary sodium, potassium, and magnesium with blood pressure; Belgian Interuniversity Research on Nutrition and Health. Hypertension, 1988; 12:594-599. 8. Simon JA, Obarzanck E, Daniels SR, Fredrick MM. Dietary cation intake and blood pressure in black girls and white girls. Am J Epidemiol. 1994; 139:130-140. 9 9. Dunne FP, Bary DG, Ferris JB, Crealy GM, and Murphy D. Changes in blood pressure during the normal menstrual cycle. Clinical and Scientific Journal of London 1991; 81:515-518. 10. Moran VH, Leathard HL, Colony J. Cardiovascular functioning during menstrual cycle. Clinical Physiology 2000; 20: 497-504. 11. Seebauer M, Fruhwith M, Moser M. Changes of respiratory sinus Arrhythmia during the menstrual cycle depend on average heart rate, Springer –Verlag. 2002; www.google.com 12. Seelig M. Cardiovascular consequences of magnesium deficiency and loss: Pathogenesis, prevalence and manifestations- magnesium and chloride loss in refractory potassium repletion. Am J Cardiol. 1989; 63: 4G-31G. 13. Seller RH, Cangiano J, Kim RF, Mendelssoln S, Brest AN, Swartz C. Digitalis toxicity and hypomagnesaemia. Am Haert J 1970; 79:57-68. 14. Woods KL, Abrams K. the importance of effect mechanism in the design and interpretation of clinical trials; the role of magnesium in acute myocardial infarction. Prog Cardiovasc Dis. 2002; 44:267-274. 15. Muir KW. Magnesium in stroke treatment. Postgrad Med J. 2002; 78:641-645. 16. Smith DA, Connick JH, Store TW. Effect of Changing Extracellular levels of Mg on Spontenous Activity and Glutamate Release in the mouse precortical slice. Br J Pharmacol 1989; 97:475-482. 17. Greene MF. Magnesium sulfate for preeclampsia . N Engl J Med. 2003; 348:275276. 10 18. Belfort MA, Anthony J, Saade GR, Allen JC. Nimodipine Study Group:A comparison of Magnesium Sulfate and nimodipine for the prevention of eclampsia. N Engl Med 2003; 348:304-311. 19. Mine FJ, Tonyz RM. Magnesium supplementation attenuates but does not prevent development of hypertension in spontaneously hypertensive rats. Am J Hypertension 1999; 12:757-765. 20. Kesteloot H. Urinary cations and blood pressure; population studies. Ann Clin Res 1984; 16:72-80. 21. White RE, Hurtle HC. Magnesium ions in cardiac function: Regulator of ion channels and second messenger. Biochem Pharmacol 1989; 38:859-867. 22. Laurant P, Tonyz RM. Physiological and Pathological role of magnesium in the cardiovascular system: Implications in hypertension. J Hypertens 2000; 18:11771191. 23. Altura BM, Turlapaty PDMVJ. Magnesium deficiency produces spasms of coronary artery: Relationship to etiology sudden death and ischeamic heart disease. Science 1980; 208:198-200. 24. Altura BM, Altura BT, Gupta PK, Ma GY, Marill GA, Kostellar AB, Zhang A. Magnesium modulates membrane lipids in vascular smooth muscle: a link to atherogenesis. FEBS Leucocytes 1997; 408:191-194. 25. Artraga E, Rojus A, Villasca P, Bianchi M. The effect of 17-β oestradiol and αtocopherol on the oxidation of LDL cholesterol from post-menopausal women and the minor effect of γ-tocopherol and melatonin. Menopause 2000; 7:112-116. 11 26. Dincer Y, Ozen E, Kadioglu P, Halemi H, Akcay T. Effects of sex hormones on lipid peroxidation in women with polycystic ovarian syndrome, health women, and men. Endocr Res 2001; 27: 309-316. 27. Seelig MS. Interrelationship of magnesium and oestrogen in cardiovascular and bone disorders, eclampsia, migraine and premenstrual syndrome. J Am Col Nut 1993; 12: 442-458. 28. Ha EJ, Smith AM. Plasma sodium and plasma erythrocyte glutathione peroxidase activity increase with oestrogen during menstrual cycle. Journal of the American College of Nutrition 2003; 22:43-51. 12 Table 1. Age, Anthropometric Data, and Estimated Menstrual Cycle Length for Subjects at Enrollment to the Study (mean±SEM, n=20). Characteristics Values Age (years) 21.6±0.2 Weight (kg) 53.7±2.3 Height (m) 1.64±0.12 BMI (kg/m2) 19.97 Cycle length (d) 28.4±0.3 Table 2. Change in estimates (mean±SEM) of Systolic and Diastolic blood pressures (SBP and DBP), Heart Rate, and Plasma Magnesium concentration (Mg2+) during the early Follicular (EF), Periovulatory (PO), and Mid-Luteal (ML) phases of menstrual cycle (n=20) GROUP SBP(mmHg) DBP(mmHg) HR(beats/min) Mg2+ (mmol/L) EF 115.15±1.28a 68.50±0.83a 69.30±1.62a 1.10±0.11a PO 119.20±1.05b 71.50±0.99b 73.60±1.71b 0.80±0.05b ML 114.10±1.23a 66.50±0.82a 70.25±1.59a 0.98±0.08a NB: Different superscript along the column indicates p<0.05 13 122 b SBP(mmHg) 120 118 116 114 a a 112 110 108 EF PO ML Menstrual Phases Fig 1. Systolic Blood Pressure Changes During Menstrual Cycle(between a and b,p<0.05) DBP (mmHg) 74 b 72 70 a 68 a 66 64 62 EF PO ML Menstrual Phases Fig. 2 Diastolic Blood Pressure Changes During Menstrual Cycle(between a and b,p<0.05) 14 HR (beats/min) 76 a 74 72 a a 70 68 66 64 EF PO ML Menstrual phases Plasma Mg (mmol/L) Fig 3. Heart Rate Changes During Menstrual Cycle(a indicates no statistical difference) 1.4 1.2 a a 1 b 0.8 0.6 0.4 0.2 0 EF PO ML Menstrual Phases Fig 4. Plasma Magnesium Changes During Menstrual Cycle(between a and b,p<0.05) 15