Safety and Efficacy of Combinations Based on Didanosine 400 mg
advertisement
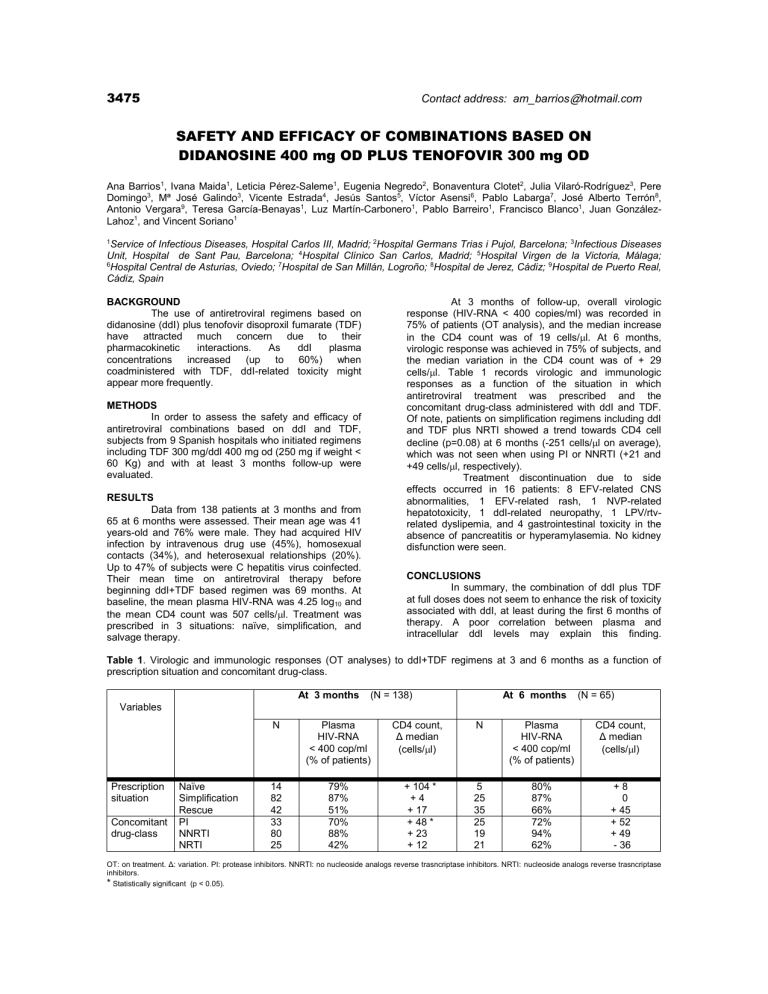
3475 Contact address: am_barrios@hotmail.com SAFETY AND EFFICACY OF COMBINATIONS BASED ON DIDANOSINE 400 mg OD PLUS TENOFOVIR 300 mg OD Ana Barrios1, Ivana Maida1, Leticia Pérez-Saleme1, Eugenia Negredo2, Bonaventura Clotet2, Julia Vilaró-Rodríguez3, Pere Domingo3, Mª José Galindo3, Vicente Estrada4, Jesús Santos5, Víctor Asensi6, Pablo Labarga7, José Alberto Terrón8, Antonio Vergara9, Teresa García-Benayas1, Luz Martín-Carbonero1, Pablo Barreiro1, Francisco Blanco1, Juan GonzálezLahoz1, and Vincent Soriano1 1 Service of Infectious Diseases, Hospital Carlos III, Madrid; 2Hospital Germans Trias i Pujol, Barcelona; 3Infectious Diseases Unit, Hospital de Sant Pau, Barcelona; 4Hospital Clínico San Carlos, Madrid; 5Hospital Virgen de la Victoria, Málaga; 6 Hospital Central de Asturias, Oviedo; 7Hospital de San Millán, Logroño; 8Hospital de Jerez, Cádiz; 9Hospital de Puerto Real, Cádiz, Spain BACKGROUND The use of antiretroviral regimens based on didanosine (ddI) plus tenofovir disoproxil fumarate (TDF) have attracted much concern due to their pharmacokinetic interactions. As ddI plasma concentrations increased (up to 60%) when coadministered with TDF, ddI-related toxicity might appear more frequently. At 3 months of follow-up, overall virologic response (HIV-RNA < 400 copies/ml) was recorded in 75% of patients (OT analysis), and the median increase in the CD4 count was of 19 cells/l. At 6 months, virologic response was achieved in 75% of subjects, and the median variation in the CD4 count was of + 29 cells/l. Table 1 records virologic and immunologic responses as a function of the situation in which antiretroviral treatment was prescribed and the concomitant drug-class administered with ddI and TDF. Of note, patients on simplification regimens including ddI and TDF plus NRTI showed a trend towards CD4 cell decline (p=0.08) at 6 months (-251 cells/l on average), which was not seen when using PI or NNRTI (+21 and +49 cells/l, respectively). Treatment discontinuation due to side effects occurred in 16 patients: 8 EFV-related CNS abnormalities, 1 EFV-related rash, 1 NVP-related hepatotoxicity, 1 ddI-related neuropathy, 1 LPV/rtvrelated dyslipemia, and 4 gastrointestinal toxicity in the absence of pancreatitis or hyperamylasemia. No kidney disfunction were seen. METHODS In order to assess the safety and efficacy of antiretroviral combinations based on ddI and TDF, subjects from 9 Spanish hospitals who initiated regimens including TDF 300 mg/ddI 400 mg od (250 mg if weight < 60 Kg) and with at least 3 months follow-up were evaluated. RESULTS Data from 138 patients at 3 months and from 65 at 6 months were assessed. Their mean age was 41 years-old and 76% were male. They had acquired HIV infection by intravenous drug use (45%), homosexual contacts (34%), and heterosexual relationships (20%). Up to 47% of subjects were C hepatitis virus coinfected. Their mean time on antiretroviral therapy before beginning ddI+TDF based regimen was 69 months. At baseline, the mean plasma HIV-RNA was 4.25 log10 and the mean CD4 count was 507 cells/l. Treatment was prescribed in 3 situations: naïve, simplification, and salvage therapy. CONCLUSIONS In summary, the combination of ddI plus TDF at full doses does not seem to enhance the risk of toxicity associated with ddI, at least during the first 6 months of therapy. A poor correlation between plasma and intracellular ddI levels may explain this finding. Table 1. Virologic and immunologic responses (OT analyses) to ddI+TDF regimens at 3 and 6 months as a function of prescription situation and concomitant drug-class. At 3 months (N = 138) At 6 months (N = 65) Variables Prescription situation Concomitant drug-class Naïve Simplification Rescue PI NNRTI NRTI N Plasma HIV-RNA < 400 cop/ml (% of patients) CD4 count, Δ median (cells/l) N Plasma HIV-RNA < 400 cop/ml (% of patients) CD4 count, Δ median (cells/l) 14 82 42 33 80 25 79% 87% 51% 70% 88% 42% + 104 * +4 + 17 + 48 * + 23 + 12 5 25 35 25 19 21 80% 87% 66% 72% 94% 62% +8 0 + 45 + 52 + 49 - 36 OT: on treatment. Δ: variation. PI: protease inhibitors. NNRTI: no nucleoside analogs reverse trasncriptase inhibitors. NRTI: nucleoside analogs reverse trasncriptase inhibitors. * Statistically significant (p < 0.05).