Standard Operating Protocol for Agarose Gel Electrophoresis
advertisement

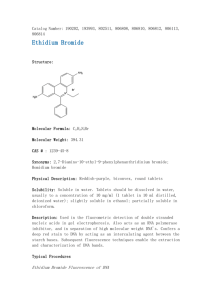
Standard Operating Protocol for Agarose Gel Electrophoresis Prepared by Jennice Lee and Shanthi Wasser Please read the complete SOP, especially the safety considerations, before starting on the experiments. Ensure that all reagents and equipment are available before starting experiment. Preparation of 1X TBE or TAE buffer 1. To make up 1X TBE (1000ml) added with ethidium bromide Dilute 200ml of 5X TBE with 800ml of RO water and add 5µl of ethidium bromide 2. To make up 1X TAE (1000ml) added with ethidium bromide Dilute 100ml of 10X TAE with 900ml of RO water and add 5µl of ethidium bromide Preparation of 1.5% TBE or TAE agarose gel 1. Wearing gloves, weigh out 1.5 g of agarose into a 250 ml pyrex conical flask. Add 100 ml of 1X TAE (or TBE) buffer with ethidium bromide added, to the flask and swirl to mix the agarose. 2. Use the microwave oven to melt the agarose. Mix the solution several times during heating and continue heating until the solution boils. CAUTION: Be careful not to allow the agarose to boil over the top of the flask. Check that all the agarose has dissolved (i.e. that no small pieces of solid agarose are visible). The flask and contents will be very hot. Wear heat resistant gloves while handling the hot flask . 3. Cool the flask under cold running water, being careful not to allow the water to enter the flask. Once the solution has cooled so that you can hold your hand on the side of the flask (Approx. 45-50°C), add 5 µl of Ethidium Bromide solution (stock solution at 10 mg/ml) and mix thoroughly but gently to avoid introducing bubbles. CAUTION: Ethidium Bromide is a powerful mutagen. Always use gloves when handling the agarose gels. Dispose of the pipette tip in a chemical waste container designated for ethidium bromide waste. Gloves in contact with ethidium bromide must be disposed in ethidium bromide waste container. 4. Pour the agarose solution into a clean, dry gel tray that has been taped at the ends with masking tape. Remove any surface bubbles with a tissue or comb, then place the comb(s) into their slots in the tray. Allow the gel to set (Approx. 30 minutes. 1 Wash out the conical flask and dispose of the residual agarose solution in the container for liquid ethidium bromide waste. 5. Carefully remove the combs (wash these and replace in the drawer), ensuring that the wells are not damaged, then the tape from the ends. Place the tray into the gel tank containing sufficient 1 x TAE (or TBE) buffer which has ethidium bromide added, such that the TAE (TBE) covers the gel to a depth of approximately 0.5 cm. Ensure that the gel is placed in the tank such that the end where the samples will be loaded is at the negative (black) end of the tank. Remove any bubbles from the wells by gently running a gloved finger over the top of the wells. Sample Loading 1. Before loading or casting a gel in an electrophoresis box always make sure that: The power supply is turned off. The voltage and current settings are turned to 0 and read 0. The leads between the power supply and the electrophoresis box are disconnected at the power supply. DO NOT place other equipment or perform procedures in close proximity to electrophoresis equipment. (Do not crowd it!!) GOOD HOUSEKEEPING IS ESSENTIAL! Always maintain adequate clearance around your apparatus. Do not permit leads to dangle below the lab bench. At the lab bench, position the power supply such that it is not necessary to reach across the apparatus to make connections to turn "ON/OFF" the power. Whenever possible set the power supply on a shelf above the gel box. 2. Add 1/5th volume of 5X agarose gel loading buffer to each DNA sample and mix by pipetting. Carefully load the DNA samples into the wells taking note of which sample is loaded in each well. As appropriate, load size and/or concentration markers in adjacent wells. If only loading samples into one set of wells, always use the lowest (nearest positive (red) electrode) set first so that other wells can be used later. 3. Place the top on the tank ensuring that the electrodes are located correctly and the positive is connected to positive etc. Connect the leads to the powerpack again checking for correct polarity, then turn on the powerpack and set the voltage at an appropriate value (usually 120 volts). Check to ensure that electrophoresis is occurring (bubbles will be generated at the electrodes). 2 4. Once the samples have run sufficiently, turn off the powerpack and wait 15 seconds before disconnecting the leads and opening the safety-interlock lid. Lift the gel from the buffer tray. Transfer to a suitable container for transportation to the dark room. CAUTION: The gel may be loose in the tray and you should support the gel at both ends to prevent it falling out of the tray. In the event of a spill or leak during a run: Do NOT touch the buffer. Turn OFF the power supply. NEVER DISCONNECT THE LEADS FROM THE ELECTROPHORESIS BOX WITH THE POWER SUPPLY TURNED ON.!!! Visualization of PCR products 1. In the darkroom, open the door to the transilluminator and carefully remove the gel from the tray. Place it on the glass surface so that the area containing your samples is in the centre. Close the door and switch on the transilluminator and camera. Alter the exposure, focus and zoom to obtain a clear image of the correct size and brightness. Freeze the image and print out the picture. CAUTION: The transilluminator produces dangerous levels of ultraviolet (UV) light. Although the system has safety interlocks to prevent exposure, always take care to ensure that the system is working properly. Failure to do so could result in exposure to UV light. Do not look directly at the UV light. Use a UV-protecting face shield or stay behind the uv-protective transilluminator shield. 2. Carefully remove the gel from the transilluminator and replace in the gel tray. Wipe down the surface with a soft tissue. Close the door and switch off the transilluminator and camera. 3. Dispose the used gel into the ethidium bromide waste container in the and the used TAE (TBE) buffer into the liquid ethidium bromide waste container with the Ethidium Bromide GreenBag disposal kit. Please refer to SOP for use of EtBR GreenBag Disposal Kit. Always wash and dry the equipment before returning it to the drawer. 3