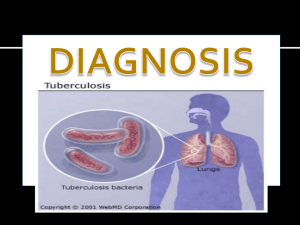
Document 7307349
advertisement

Ph.D. THESES GHAZALA M. FURGANI 2006. GNOTOBIOLOGICAL ANALYSIS OF ENTOMOPATHOGENIC NEMATODE / BACTERIUM SYMBIOTIC COMPLEXES Ph.D. THESES GHAZALA M. FURGANI SUPERVISOR: DR. ANDRÁS FODOR Accredited PhD program: Molecular, classical and evolutionary genetics (Head: Prof. Gábor Vida, Full Member of the Hungarian Academy of Sciences) DEPARTMENT OF GENETICS, FACULTY OF NATURAL SCIENCES EÖTVÖS LORÁND UNIVERSITY BUDAPEST, HUNGARY BUDAPEST, 2006. 2 CONTENT INDEX OF FIGURES ................................................................................................................................ 6 INDEX OF TABLES ................................................................................................................................. 7 1. INTRODUCTION ..................................................................................................................................... 8 1.1. REVIEW OF THE LITERATURE ON ENTOMOPATHOGESIS IN NEMATODES (EPN) AND BACTERIS (EPB)...................................................................................................................................... 8 1.1.1. Taxonomic position of the EPN species ...................................................................................... 8 1.1.1.1. Taxonomic position of the EPN species within Phylum Nematoda ..................................................... 9 1.1.2. The Steinernema genus .............................................................................................................. 10 1.1.3. The Heterorhabditis genus ........................................................................................................ 12 1.1.4. The biological background of insect pathogenesis caused by EPN/EPB symbiotic complexes 14 1.1.5. Entomopathogenic symbiotic bacteria ....................................................................................... 15 1.1.6. Mutualism between entomopathogenic nematodes and bacteria ............................................... 18 1.2. OBJECTIVES AND RATIONALE .................................................................................................. 21 2. MATERIALS AND METHODS............................................................................................................ 23 2.1. ENTOMOPATHOGENIC NEMATODE AND BACTERIA USED IN THIS STUDY .................. 23 2.1.1. EPN STRAINS .......................................................................................................................... 23 3.1.2. EPB STRAINS .......................................................................................................................... 24 2.2. METHODS AND TECHNIQUES .................................................................................................... 25 2.2.1. MICROBIOLOGICAL METHODS.......................................................................................... 25 2.2.1.1. SOLUTIONS AND MEDIA .............................................................................................................. 25 M9 a buffered physiological salt solution for nematodes ........................................................................... 25 Liquid (or solid) LB media (Luria Bertani medium) (without or with agar): ............................................. 25 Liquid (or solid) TSY media (without or with agar): ................................................................................. 25 Liquid (or solid) NA media (without or with agar): ................................................................................... 26 NBTA an indicator medium ....................................................................................................................... 26 MacConkey Indicator Agar ........................................................................................................................ 26 Wouts” agar media ..................................................................................................................................... 26 2.2.1.2. METHODS ......................................................................................................................................... 27 ISOLATION OF THE SYMBIOTIC BACTERIA FROM DAUER JUVENILES .................................... 27 FREEZING OF BACTERIA FOR LONG-TERM STORAGE ................................................................. 27 SURFACE STERILIZATION OF INFECTIVE DAUER JUVENILES ................................................... 28 2.2.2. METHODS FOR GNOTOBIOLOGY ...................................................................................... 28 3. RESULT AND DISCUSSION ................................................................................................................ 30 3.1. ANTIBIOTICS PRODUCTION OF THE EPB STRAINS ....................................................... 30 3.1.1. ISOLATION OF EPB SYMBIONTS DIRECTLY FROM DAUER LARVAE OF STEINERNEMA SPECIES .................................................................................................................. 30 3 3.1.2. ANTIBIOTICS PRODUCTION OF DIFFERENT XENORHABDUS AND PHOTORHABDUS STRAINS TESTED ON BACILLUS CEREUS ................................................................................... 31 3.1.3. THE EFFECT OF ANTIBIOTIC PRODUCTION ON OTHER ENTOMOPATHOGENIC BACTERIA ......................................................................................................................................... 34 3.1.4. EFFECTS OF THE XENORHABDUS ANTIBIOTICS ON ERWINIA AMYLOVORA ............. 39 3.1.4.1. EFFORTS TO CONTROL THE FIRE BLIGHT DISEASE IN APPLE ORCHARDS ..................... 39 3.1.4.2. LABORATORY TESTS ON ERWINIA AMYLOVORA ..................................................................... 40 3.2. FERMENTATIVE PRODUCTION, CHEMICAL IDENTIFICATION AND APPLICATION OF EMA ANTIBIOTICS ............................................................................................................................... 43 3.2.1. FERMENTATION OF THE EMA STRAIN AND TESTING THE ANTIMICROBIAL ACTIVITY OF THE FERMENTATION SOUP ................................................................................ 43 3.2.2. ISOLATION OF COMPOUNDS OF ANTIMICROBIAL ACTIVITY FROM FERMENTOR LIQUID CULTURE OF XENORHABDUS SP. EMA STRAIN ......................................................... 44 3.3. THE EMC STRAIN .......................................................................................................................... 50 3.3.1. THE PHENOTYPIC CHARACTERIZATION OF THE EMC STRAIN ................................. 50 3.3.1.1. COLONY MORPHOLOGY AND SWARMING BEHAVIOR ......................................................... 50 3.3.2. EXO-CRYSTAL PRODUCTION ............................................................................................. 51 3.3.3. ANTIBIOTICS PRODUCTION OF EMC ................................................................................ 57 3.3.4. CONCLUSIONS ....................................................................................................................... 58 3.4. GNOTOBIOLOGY .................................................................................................................... 59 3.4.1. RESULTS IN GNOTOBIOLOGY ............................................................................................ 59 3.4.2. CONCLUSIONS ....................................................................................................................... 63 3.5. FIRST ATTEMPTS ON LABORATORY FERMENTATION OF A NEW STEINERNEMA ISOLATE ................................................................................................................................................. 64 SUMMARY ........................................................................................................................................ 64 3.5.1. INTRODUCTION ..................................................................................................................... 64 5.5.2. MATERIALS AND METHODS .............................................................................................. 64 3.5.3. ISOLATION AND CHARACTERIZATION OF EPB SYMBIONTS FROM SOME NEW STEINERNEMA STRAINS OF LONG DAUER PHENOTYPE ........................................................ 65 3.5.1.1. PHENOTYPIC CHARACTERIZATION OF THE FRESHLY ISOLATED BACTERIAL SYMBIONTS .................................................................................................................................................. 65 3.5.1.2. PHOSPHOLIPASE (LECITINASE TEST ON YOLK AGAR) ......................................................... 65 3.5.1.3. PROTEOLYSIS ON GELATIN AGAR (FRAZIERS’S METHOD) ................................................. 66 3.5.1.4. ANTIBIOTICS PRODUCTION ......................................................................................................... 66 3.5.1.6. AMPICILLIN SENSITIVITY ............................................................................................................ 66 3.5.1.7. GNOTOBIOLOGICAL TESTS ......................................................................................................... 66 3.5.2. RESULTS AND DISCUSSION ................................................................................................ 67 3.5.3. LABORATORY FERMENTATION OF THE MOROCCO STRAIN ..................................... 69 3.5.4. CONCLUSIONS ....................................................................................................................... 70 3.6. MOLECULAR, GENETIC AND GNOTOBIOLOGICAL IDENTIFICATION OF NEW STEINERNEMA ISOLATES .................................................................................................................... 72 4 SUMMARY ........................................................................................................................................ 72 3.6.1. INTRODUCTION ..................................................................................................................... 72 3.6.2. MATERIALS AND METHODS .............................................................................................. 73 3.6.3. RESULTS AND DISCUSSION ................................................................................................ 74 4. SUMMARY ............................................................................................................................................. 79 5. REFERENCES ........................................................................................................................................ 80 6. ACKNOWLEDGEMENTS .................................................................................................................... 84 5 Index of Figures Fig. 1: Life cycle of Steinernema species Fig 2: The life cycle of the Heterorhabditis species Fig. 3: The biological role of the EPB symbiont Fig. 4: Isolation of EPB symbionts from EPN infective dauer juveniles Fig. 5. Antibiotics production of EMC (left) and X. sp. (bibionis) (right) determined by using B. cereus or B. subtilis as indicator strains. Fig 6. A qualitative and quantitative comparison of the antibiotics produced by different Xenorhabdus strains Fig. 7. Demonstration of Bibionis inhibiting the growth of Photorhabdus temperata ssp. temperata strain HL81. Fig. 8: Outlines of laboratory tests on Erwinia amylovora Fig. 9: Tests on EMA antibiotics on E. amylovora on Petri plate, liquid culture and phytotrone. Fig. 10A: Fractionation of the biologically active compounds adsorbed to charcoal. Fig. 10B: Fractionation of the biologically active compounds adsorbed to DOWEX 50 Fig.11: Swarming phenotype of EMC. The color of the colonies is also unusual. Fig. 12: The metallic-like crystals appear not only in the media but also on the surface of the older colonies in agar plates Fig. 13 The exo-crystal production of two isolates, EMC and EMD. Fig 14 A Crystal production of EMC in liquid minimum media Fig 14 B Crystal production of EMC in liquid minimum media Fig. 15A. Light microscopic picture of the isolated crystal. 125X magnification. Fig. 15B. Light microscopic picture of the isolated crystal. 250X magnification Fig. 15C. Light microscopic picture of the isolated crystal. 250X magnification Fig. 16. EM picture and image analysis of the exo-crystal Fig. 17 The proof of the cytotoxic activity of the antimicrobial compound of EMC and EMA. Fig. 18. Effect of EMC antibiotics on the colony formation of Phytophtora sp. Fig. 19: The new Xenorhabdus isolates (Morocco, SP2, Italy, Slovak) and control (X. nematophila (AN6/1), X. poinarii (DSM 4766) strains on Luria Broth (LB) agar plates Fig. 20: Lecitinase activities of my new isolates. Control: DSM 4766 (X. poinarii). Fig. 21: Lipase activities of my new Xenorhabdus isolates Fig. 22: In the article: (Fig. 6). Antibiotics activities of my new Xenorhabdus isolates Fig 23. Ampicillin resistance of the new Xenorhabdus isolates Fig. 24: The laboratory fermentation unit. Fig 25: GenePhore PCR-RFLP Analysis 1. Fig. 26: GenePhore PCR-RFLP Analysis 2 Fig. 27: GenePhore Analysis 3 9 11 16 31 32 35 36 41 45 47 49 51 52 53 54 55 56 56 57 57 58 59 69 69 70 71 71 72 79 80 81 6 Index of Tables Table 1. The Steinernema strains in our stock collection and their natural symbionts 21 Table 2. The Xenorhabdus strains used in this study 22 Table 5 Antibiotics production of the EPB strains Table 6: Antagonism of Xenorhabdus vs. Photorhabdus. I Table 7. Antagonism of Xenorhabdus vs. Photorhabdus. II Table 8. Antagonism of Xenorhabdus vs. Photorhabdus. III. Table 9. Antagonism of Xenorhabdus vs. Photorhabdus. IV Table 10: The effectivity of EPB antibiotics on 10 E. amylovora isolates (by comb test) Table 9: The stability of the water solution of the EMA antibiotics at + 4 Co after half a year storage Table 11: Comparison of the biological activities of the compounds with antimicrobial activity on B. subtilis Table 12. Summary of the gnotobiological tests of Heterorhabditis / Photorhabdus complexes (data of E. Böszörményi and Katalin Lengyel) Table 13: Growth of S. carpocapsae and S. feltiae strains on symbionts of S. feltiae and closely related species Table 14: Growth of S. carpocapsae and S. feltiae strains on Xenorhabdus symbionts of S. carpocapsae and related species Table 15: Growth of S. carpocapsae and S. feltiae strains on Xenorhabdus symbionts of other Steinernema species of short dauer phenotype Table 16: Growth of S. carpocapsae and S. feltiae strains on Xenorhabdus symbionts of other Steinernema species of long dauer phenotype Table 17: Growth of S. carpocapsae and S. feltiae strains on Xenorhabdus symbionts of taxonomically undetermined Steinernema species Table 18: (In the paper: Table 1). Growth and propagation of Steinernema strains on each others’ symbionts Table 19: (In the article: Table 2) The results of cross breeding tests 33 37 38 39 40 42 46 50 60 61 62 63 64 64 77 78 7 1. INTRODUCTION 1.1. REVIEW OF THE LITERATURE Many types of associations exist between nematodes and insects ranging from phoresis to parasitism and pathogenesis. The families Steinernematidae, Neosteinernematidae and Heterorhabditidae are unique because they are the only nematodes which have developed the ability to carry and introduce symbiotic bacteria into the body of insects, they are the only insect pathogens with a host range which includes the majority of insect orders and families and they can be cultured in large scale on or in artificial solid or liquid media (Poinar, 1990). The nematodes that serve as vectors of pathogenic bacteria show considerable potential in biological control of insect pests. They are called entomopathogenic nematode (EPN) and used as biological control agents. They are insect parasitoids, since the immature forms of EPN species develop at the expense of one host individual. They are also ecologically similar to both parasitoids and arthropod predators because the host individual is eventually killed (Kaya et. at 1985). EPN species belonging to the different families possess many qualities that make them excellent biological control agents. They have a broad host range, can easily be mass produced, possess the ability to seek out their host, kill their host rapidly and are environmentally safe. These attributes are combined with others like the easy application by using standard spray equipment, compatibility with many chemical pesticides and no evidence of mammalian pathogenicity (Ehlers and Peters, 1995, Ehlers and Hokanean, 1996, Boemare et al, 1996). The soil environment is one that offers an excellent site for insect nematode interactions, because more than 90% of insect pests spend part of their life cycle in the soil, and the soil is the natural reservoir of steinernematid and heterorhabditid nematodes. 1.1.1. Taxonomic position of the EPN species When considering the number of species of Animal Kingdom, Phylum nematoda is the second following Arthropoda. According to estimates, several hundred thousands species belong to this taxonomic group. They inhabit mainly soil, but also freshwater and sea. The majority of them are neutral concerning their relation with mankind, mostly 8 feeding on soil bacteria or decomposing organic material. A group of them is harmful as plant pathogens, while others cause tropical diseases, the EPN species are beneficial and commercially useful nematodes. 1.1.1.1. Taxonomic position of the EPN species within Phylum Nematoda The classification Phylum Nematoda (after Andrássy, 1976) Class Adenopohora Order Mermithida Suborder Mermithina Superfamily Mermithoidea Family Mermithidae Family Tetradonematidae Class Secernentea Order Rhabditida Suborder Rhabditina Superfamily Diplogasteroidea Family Diplogasteridae Superfamily Rhabditoidea Family Steinernematidae Family Neosteinernematidae Family Heterorhabditidae Family Steinernematidae Genus: Steinernema Travassos, 1927 Type strain: Steinernema carpocapsae Species that was discovered first: Steinernema kraussei (Steiner, 1923, Travassos, 1927). Most important species: S. carpocapsae (for general use); S. feltiae (controlling mushroom flies), S. glaseri, S. scarabeaiae (both controlling scarabs); S. scapterisci (to control mole cricket and cockroaches); S. abassi, S. Riobravia (used in hot conditions). Other species involved in this study: S. rarum, S. bicornutum, S. serratum, S. cubanum, Family Heterorhabditidae Genus: Heterorhabditis Poinar, 1976 Type strain: Heterorhabditis bacteriophora Poinar, 1976. Other species involved in this study: H. megidis (see review of Adams, 2002); H. downesii (Griffin et al., 1997); H. marelata (Liu et al., 1994), H. indica (see review of Adams, 2002) The EPN species are phylogenetically close to Caenorhabditis elegans (Brenner, 1974; The C. Elegans Consortium, 1998), which provides the possibility to adopt the information gathered by the C. elegans researchers to EPN. The EPN species belong to three families, namely Steinernematidae, Neosteinernematidae and Heterorhabditidae. Each EPN family is comprised by a single genus. In the family Steinernematidae the species have been more or less unambiguously 9 identified by their morphological differences, supplemented by crossbreeding tests. Despite some problems with misidentification and nomenclature, there has not been too much difficulty in species recognition. In contrast, when working with Heterorhabditis, it is complicated to obtain crossbreeding data and there have been some difficulties in defining taxonomically useful morphological characters (Ilona Dix, personal communication). The taxonomy of Heterorhabditis spp. will likely be based on morphological analysis and construction of phylogenetic and derived classification system (Curran 1990, Stock et al, 1996 Adams et al, 1998). The major purpose of molecular techniques in EPN taxonomy is the identification of sibling species, subspecies, and other intraspecific groupings. In addition, it contributes to understanding the biology and evolution of EPNs. This role may become of critical importance in identifying species and isolates for registration, quarantine and proprietary protection purposes. This study mainly focuses on Steinernema species and their symbionts. 1.1.2. The Steinernema genus The first Steinernema species was named by G. Steinernema, and the species was Steinernema kraussei. He named the nematode, isolated in Germany, Aplectana kraussei (Poinar, 1990). In 1927, Travassos determined a new genus, Steinernema for the species. However, apparently two different species are included in the original description of S. kraussei. Thus that species is presently considered as a species inquirenta, and the name “kraussei” as nomen dubium. In 1929, Steiner erected the genus Steinernema. In 1934 Filipjev noted the resemblance between the two genera and placed them in subfamily Steinernematidae. This subfamily was erected to family level by Chitwood and Chitwood in 1937. Both genera, Neoaplectana and Steinernema, were considered valid, but all recognized species were placed in the former genus because it was more completely defined and the type species (glaseri) was intensively studied in the 1930 and 1940s. Species belonging to Steinernema are obligate entomopathogenic nematodes that are capable of infecting a wide variety of insects. As shown on Figure 1., they have a life cycle including a third stage alternative, the so called infective dauer juvenile (IJ). This is the only developmental variant, which can be found outside the insect, and this stage is the vector for the entomopathogenic bacterial symbiont (belonging to the Xenorhabdus genus) that eventually kills the insect after brought to its body cavity and hemolymph. Each Steinernema species, and even each strain of the same species, carries different 10 Xenorhabdus strains. The bacterium is an obligate symbiont of the steinernematid nematode. The symbiont has a primary and a secondary variant form. The primary (10) – secondary (20) transition is spontaneous and is usually a “one-way street.” Only the primary form is capable to establish an intimate symbiosis with the nematode, probably based on a signal transduction mechanism between some gut cells of the alimentary tracts of the IJ and the 10 of the EPB symbiont. Therefore the EPN / EPB symbiosis is developmentally controlled from both sides. The infective dauer juvenile is capable of surviving in the soil, entering the body cavity of a host and then developing into a female or male. After two or three generations in the host the nematodes the infective juvenile stage appears again, leaves the host and begins searching for a new host. The infective stage juvenile is an alternative third stage juvenile, often still inside the second stage cuticle. The mouth and anus are closed, the pharynx and intestine is collapsed. In every (but one) Steinernema species the IJs develop in to an amphimictic female or a male but never into hermaphrodites (see Fig. 1.). The EPN species have developmental larvae stages (J1 – J4). An alternative developmental pathway leads to IJ, which leaves the insect host and searches for another one. The choice between the two pathways is made at the end of the first stage is based on chemical stimulus. When the nematodes are crowding, the concentration of a pheromone (DRIF) overpasses the threshold concentration, which, just like in case of C. elegans, induces dauer (IJ) formation (Fodor et al., 1994). The number of the IJ is correlated with the number of nematodes present in the insect cadaver. At this stage there is usually food storage and the “food signal” competes with the pheromon (Golden and Riddle, 1982, 1984, Riddle 1988). The reduction of food does not only induce IJ formation, but also blocks the recovery from the IJ stage. A pheromone is a chemical stimulus to the late J1 stage, which responds by developing into the alternative (J2D – dauer) pathway. The intimate relation of the nematode and bacterium happens in the gut of the IJ through an active retention of the bacteria by the cells of the vesiculum or bursa intestinalis (in steinernematids). (No specialized cells were found for retaining the EPN symbiont in Heterorhabditis. Maybe the whole intestine is responsible for the prokaryotic / eukaryotic cell – cell communication). Almost all phase 1 (1o) bacterial cells are consumed prior to the dauer larva formation. Consequently, the primary stage bacteria and the dauer larvae form monoxenic symbiotic combinations. When the IJ enters the body of the insect, it releases the bacteria from its alimentary tract into the haemocoel. 11 After the death of the larvae the whole insect becomes monoxenic in which the nematode and its symbiont are present. Fig. 1: Life cycle of Steinernema species LEGEND to Fig. 1. All but one Steinernema species are amphimictic. There sex is determined by XX / X0 mechanisms. There are males and females. In the insect cadaver the fertilized eggs hatch as first larval juvenile (J1). These animals molt into J2, J3, J4 and adults, respectively. When the nematode population is overcrowded (usually in the third generation in the nature), a chemical signal (called dauer inducing pheromone, DRIF) passes a threshold concentration which induce in the available J1 larvae an alternative developmental pathway. These worms grow to predauer (J2d) and then infective dauer juveniles (IJ). The IJ worms represent a non-feeding, non-ageing developmental variant. They leave the cadaver and search for another insect hosts. They then molt to J4, release a few cells of the primary form of their natural Xenorhabdus symbiont. The bacteria kill the insect by their toxins and then colonize the insect cadaver. The bacteria convert the insect tissues consumable for the nematodes, serve as food as well and even protect the monoxenic culture by producing antimicrobial compounds. 1.1.3. The Heterorhabditis genus The Heterorhabditidae family, Heterorhabditis genus was described in 1975 with H. bacteriophora as the type species (Poinar, 1975). These are obligatory parasitic rhabditids with biology similar to the Steinernematids. The Heterorhabditis species have also a similar, but not identical life cycle including a third alternative - infective dauer juvenile (IJ). An important difference between the two genera is the mechanism of sex determination. Heterorhabditids have alternative life cycles with hermaphrodites and amphimictics. As for the history of Heterorhabditidae, the nematode H. heliothidis was originally described as a member of the family Steinernematidae, but later on it was synonymized with Heterorhabditis bacteriophora (Poinar, 1990). Each IJ develops into a 12 hermaphrodite (anatomically, just like C. elegans, the female is producing both sperm and ova). However, the second generation consists of automictic hermaphrodites and amphimictic (female and male) adults (Dix et al., 1992). This heterogamy, and the occurrence of amphimictic and automictic adults make the life cycle and sex determination of heterorhabditid nematodes very complex. Fig 2: The life cycle of the Heterorhabditis species LEGEND to Fig 2.Each Heterorhabditis species has an automictic and an amphimictic life cycle. The mechanism of sex is determination is unknown. From the IJs 100 % self-fertilizing hermaphrodites develop. Their progenies are of 50% males and females. In the insect cadaver the fertilized eggs hatch as first larval juvenile (J1). These animals molt to J2, J3, J4 and amphimictic adults, respectively. When the nematode population is overcrowded (usually in the third generation in the nature) a chemical signal (called dauer inducing pheromone, DRIF) passes a threshold concentration, which induce an alternative developmental pathway in the available J1 larvae. These worms grow to predauer (J2d) and then infective dauer juveniles (IJ). The IJ worms represent a non-feeding, non-ageing developmental variant. They leave the cadaver and search for another insect host. They then molt to hermaphroditic J4, release some cells of the primary form natural Photorhabdus symbiont. The bacteria kill the insect by their toxins and then colonize the insect cadaver. The bacteria convert the insect tissues consumable for the nematodes, serve as food as well as, and protect the monoxenic culture by producing antimicrobial compounds. In the last 10 years new species have been determined by morphology (Poinar et al, 1994, Luanda Berry 1995, Stock et al, 1996, Adams et al, 1998 Blaxter et al, 1998). The Heterorhabditis species are all obligate EPNs, capable of infecting a wide variety of insects. Alternative third-stage infective juveniles (IJ), are often enclosed in a second stage cuticle. Cells of a symbiotic bacterium (Photorhabdus spp.) occur in the infective juvenile’s alimentary tract. Infective juveniles are capable of surviving adverse 13 environmental conditions that would be impossible for all other developmental variants (see Fig. 2). The IJs enter the body cavity of host or penetrate directly throughout the insect cuticle. After infecting and releasing the symbiotic bacteria into the haemocoel, they develop into J4 and then the adult stage, a self - fertilizing hermaphroditic female. Thus in infection, a single hermaphroditic Heterorhabditis IJ is sufficient to initiate nematode development and proliferation within the haemocoel (Zioni et al, 1992). These first generation Heterorhabditis hermaphrodites give rise to a second generation, which consists of amphimictic females and a few hermaphrodites (Fig. 1.). There are large differences in the percentage of male progeny produced by the first generation hermaphrodites in different species and strains of Heterorhabditis (Dix et al, 1992). Hermaphroditic and amphimictic populations are found only inside infected cadavers in nature (Poinar, 1992). Heterorhabditis spp. have been recognized by molecular identification as H. bacteriophora, H. indica, H. marelata, H. megidis, H. zealandica, and H. argentinensis (Adams et al., 1998). It was reported that Heterorhabditis strains belonging to the North Western European (NWE) group were considered as H. megidis when compared to the original OH1 H. megidis strain (Poinar et al., 1987). Heterorhabditis strains isolated in Ireland and classified as Irish type are represented by strains such as K122 or M145 (Dix et al., 1992, Griffin et al., 1994). The new name of the species is Heterorhabditis downesii (Griffin et al., 1994). 1.1.4. The biological background of insect pathogenesis caused by EPN/EPB symbiotic complexes Infection by both Steinernema and Heterorhabditis is initiated by a third stage juvenile, which is morphologically and physiologically adopted to remain in the environment for a prolonged period (without taking nourishment). The IJ stage is the only survival stage in the life cycle of these nematodes. Morphological adaptations for this survival period include a collapse of certain body tissues. The alimentary tract essentially nonfunctional, because the walls of the intestine and pharynx have closed together, thereby greatly reducing the lumen of the digestive tract. The mouth and anus are closed. Symbiotic bacteria (Xenorhabdus or Photorhabdus), which play an important role for nutrition inside the host, are found in the alimentary tract of the infective stage. In Steinernema, the great majority of the bacteria are found in the modified ventricular 14 portion of the intestine. In Heterorhabditis .Photorhabdus is found in this location as well, but can also occur throughout the intestine and in the pharyngeal lumen. 1.1.5. Entomopathogenic symbiotic bacteria The entomopathogenic bacteria that are symbiotic with the nematodes were first placed in the genus Xenorhabdus. On the basis of physiological and genomic analysis data, it was later proposed that the bacteria, which emit light (X. luminescens), should be given genus status and this taxon become designated Photorhabdus luminescens. The symbiotic nematode partner is Heterorhabditis spp. The Xenorhabdus species are obtained from nematodes belonging to the genus Steinernema (Szállás et. al., 1997). Xenorhabdus and Photorhabdus spp. are Gram negative gamma Protobacteria that form entomopathogenic symbiosis with soil nematodes. Their life cycle involves a symbiotic stage in which they are carried in the gut of the nematode and a pathogenic stage in which the insect prey is killed by the combined action of the nematode and the bacteria (Forst et. al., 1996). As mentioned above, the bacteria are carried in the intestine of the infective (dauer) juvenile stage of nematode. The nematode enters the digestive tract of the larvae of diverse insects and penetrates into the haemocoel. Nematodes also enter through the respiratory spiracles or by penetrating directly throughout the insect cuticle (Forst et. al., 1997). Upon entrance into the haemocoel the nematodes release the bacteria into the hemolymph. Within the haemocoel of larva, the bacteria grow to stationary phase while the nematodes develop and sexually reproduce. In the stationary phase, bacteria break down the host by secreting several extracellular products including lipase(s), phospholipase(s), protease(s), and several different broad spectrum antibiotics (Forst et. al., 1997). These products are believed to be secreted into the insect hemolymph. When bacteria enter stationary phase conditions, the degrading enzymes break down the macromolecules of the insect cadaver to provide the nematode with the nutrient supply, while the antibiotics prevent the contamination of the cadaver with other microorganisms. Cytoplasm inclusion bodies - composed of highly expressed crystalline proteins - are also produced by both bacteria during the stationary phase (Couche et. al., 1987). Nematode reproduction is optimal when the natural symbiont dominates the microbial flora, suggesting that the bacteria can serve as food source and provide essential nutrients that are required for efficient nematode proliferation (Akhurst et. al., 15 1986). This stage of the bacteria which provides several compounds for the nematodes, is named Phase 1 or primary phase (1o). The bacteria may also enter into Phase 2 or the secondary phase (2o). Both phases can serve as food supply, but only the Phase 1 cells produce antibiotics. A possible explanation of the primary - secondary phase shift may influence the survival of the nematode (Poinar, 1978), especially in laboratory or fermentation conditions. It should be emphasized, that although both phase variants are taken up by the nematodes and used as food, only the Phase 1 cells are retained by infective dauer juveniles and exists as symbionts for a longer period of time. In case of steinernematids several other bacteria including (E. coli) could be used as nutrients. Freshly hatched first stage (J1) juveniles can develop up to adult age on E. coli, but the adults are infertile (Vanfleteren and Fodor, unpublished) if the Phase 1 cells of the symbiont are not present. When Steinernema spp. dauer larvae are transferred into a culture of either a non - symbiotic or Phase 2 cells of their associated bacteria, they may recover and grow to low fertility or infertile adults. When growing steinernematids in axenic media or fertilized chicken eggs, they may grow, but poorly produce infective dauer juveniles. Heterorhabditids are different. They grow and reproduce exclusively on the Phase 1 cells of their symbionts (Fodor et al., unpublished). During the final stage of development, the nematode and bacteria re-associate and the nematode subsequently develops into its infective dauer juvenile (IJ) stage. The IJ carries the bacteria in its intestinal tract then emerges from the insect carcass and searches a new insect host. All the Xenorhabdus isolates studied so far have been obtained from nematodes harvested from soil samples. Free-living forms of the bacteria have not been isolated from soil or water (Forst et. al., 1997). The above findings suggest that the symbiotic association may be essential for the survival of the bacteria in the soil environment. It is a little bit contradictory to the fact, that there is no problem of growing Xenorhabdus spp. or Photorhabdus spp. on conventional bacterial media in the laboratory. The only known thing about these symbiotic bacteria is that they are proline auxotrophs (Nealson et al., 1990). Except for a very few known cases, the bacteria in turn are essential for effective killing of the insect host and they are required for the nematode to complete its life cycle. There is only one known Steinernema species (isolated from Azores), which does not have any bacterial symbiont. This Steinernema glaseri strain is in facultative symbiosis with X. poinarii. In any population of this nematode strain one can always find nematodes without bacteria. 16 On the other hand, some insects are resistant to S. glaseri, (when the bacteria are not present), but not to the symbiotic complex (Ray Akhurst, Ralf - Udo Ehlers, personal communication). Both bacterial genera belong to the Enterobacteriaceae but are nitrate reductase negative. Photorhabdus are catalase positive and are the only known terrestrial bacteria able to emit light similar to that of the marine bioluminescent bacteria. The taxonomy of Xenorhabdus and Photorhabdus has been discussed elsewhere (Boemare et al., 1993, Akhurst and Boemare, 1994, Boemare et al.,1993) used a method of total genomic DNA hybridization to re-evaluate the designation of Xenorhabdus species which had been determined by classical methods of bacterial taxonomy. Their work confirmed that X. beddingii, X. nematophila, X. bovienii and X. poinarii are valid species whereas the fifth species, X. luminescens was transferred to a new genus, as Photorhabdus luminescens. Photorhabdus and Xenorhabdus spp. are facultative anaerobic Gram negative rods, non sporulating oxidase negative chemoorganotrophic heterotrophs with respiratory and fermentative metabolisms. They belong to the family Enterobacteriaceae in groups 5 and 1 (Akhurst, 1980, Boemare et al, 1993, Hoot et al, 1994). The general features of the life cycles of these bacteria are quite similar. Xenorhabdus and Photorhabdus spp. are carried as symbionts in the intestine of infective juvenile stage of nematodes belonging to the families Steinernematidae and Heterorhabditidae, respectively (Akhurst, 1993, Forst and Nealson, 1996, Poinar, 1990). The bacterial strains belonging to the Photorhabdus genus are usually symbionts of entomopathogenic nematode (EPN) strains. The only exceptions are those isolated from clinical material (human wounds, Colepicolo et al, 1989) and have recently been recorded as the novel species P. asymbiotica (La Saux et al, 1999). Photorhabdus species other than those belonging to P. asymbiotica are carried in the gut of the infective dauer juveniles (IJ). While Xenorhabdus spp. and Photorhabdus spp. are similar in numerous characteristics, they differ in several salient features. Xenorhabdus spp. is specifically found associated with EPNs in the group Steinernematidae, while Photorhabdus spp. only associated with the nematode group Heterorhabditidae. A primary property distinguishing Photorhabdus from Xenorhabdus spp. is the ability of the former to emit light under stationary phase culture conditions and in the infected host insect (Poinar et 17 al., 1980). On the other hand, Xenorhabdus is catalase negative which is an unusual property for bacteria in the Enterobacteriaceae family (Boemare, 1990 Farmer 1984). 1.1.6. Mutualism between entomopathogenic nematodes and bacteria As discussed above, the bacteria need the nematode as a vector to bring them from one insect hemolymph to the next. The bacteria do not survive well in soil (Poinar et al., 1980), and are generally not pathogenic for insect when ingested. The nematode needs the bacteria to kill the insect. Heterorhabditids are not able to kill an insect without their symbiont (Han et al., 1990) and the steinernematids are less pathogenic without their symbiont (Akhurst, 1986, Dunphy et al., 1985, Ehlers et al., 1990). The nematodes help the bacteria by suppressing the antibacterial immune system of the insect (Götz et al., 1981). The nematodes also need the symbiont to get access to right nutrient. Again, Heterorhabditids are more specific in this process than Steinernematids. Steinernematids can be cultured in vitro on a complex medium without bacteria while Heterorhabditids can be grow only in the presence of their own symbiont or a very closely related symbiont strain (Luan et al., 1993). The bacteria produce antibiotics to inhibit growth of other microorganisms in the insect cadaver (Akhurst, 1982). Although adult and developing nematodes can digest bacterial cells, the bacteria are not digested inside the infective juveniles, since they need the bacteria to kill a new host. The close relationship between nematodes and bacteria is also illustrated by the fact that most nematode species are associated with a single bacterium species (Akhurst and Boemare 1988, Smith and Ehlers, 1991). Steinernema carpocapsae is associated with Xenorhabdus nematophila, S. glaseri with X. poinarii, S. feltiae and S. affinis with X. bovienii, etc. Currently all symbionts of Heterorhabditis spp. are classified as Photorhabdus but different species. The biological role of the EPB symbiont is summarized in Fig. 3. The symbiont demolishes the immune system of the insect and kills it by toxins. The EPB cells released into the haemocoel convert the insect tissue consumable for the nematode by their exoenzymes. Finally, they protect the monoxenic system against soil microorganisms by producing antimicrobial compounds of large spectrum. 18 Fig. 3: The biological role of the EPB symbiont LEGEND to Fig. 3.. The symbiont demolishes the immune system of the insect and kills it by toxins. The EPB cells released into the haemocoel convert the insect tissue consumable for the nematode by their exoenzymes. Finally, they protect the monoxenic system against soil microorganisms by producing antimicrobial compounds of large spectrum. Entomopathogenic bacteria belonging to the genera of Xenorhabdus and Photorhabdus are known to produce several compounds with antimicrobial activity, effective against large spectrum of Gram (+) and Gram (-) bacteria and against several fungi. The antibiotics production profile of the different strains is different. Entomopathogenic nematodes can be used to control several agricultural insect pests. All known species can be used successfully against most Lepidoptera especially if some developmental stage is in the soil. S. feltiae is used against mushroom (Sciaridae) flies in the US and several EU countries. They are also effective against Othyorhynchus species. In Hungary, S. carpocapsae Mexicana and All strains proved to be effective against Colorado potato beetle (Leptinotarsa decemlineata) larvae in heavily irrigated conditions (Fodor and Sáringer, 1986). S. glaseri and several Heterorhabditis strains are widely used against scarabid grubs with successes all over the world. We have recently tested the effect of Heterorhabditis sp. HU86 in our laboratory with promising results. The agricultural potential of the toxins and antibiotics produced by entomopathogenic bacteria is a new and growing challenge. 19 The natural product of biologically active materials produced by the EPB strains (as well as of their biological role) is summarized in Fig. 3. Both the primary and the secondary forms of each EPB strains produce toxins of protein nature (see the review of french-Constant, 2003). They cause septicemia after being released to the blood of the infected insects while others exert oral toxic activity as well. The phase 1 variant bacteria also produce proteases and other exo-enzymes aiming it inactivate the immune system of the insect hosts. (Some exo-enzymes are also produced by the phase 2 forms as well). Again, phase 1 variant bacteria produce antibiotics effective against almost all Gram positive as well as a large number of Gram negative bacteria. It is not known whether the antifungal activity of the fermentation soup of the phase 1 EPB strains are due to the same molecules as the antibacterial activity. What is known, that (i) only the primary variant produces compounds of antimicrobial activity, and (ii) the spectrum and the amount of these compounds differs from one species to another. The primary forms of each EPB strain also produces intracellular crystals of protein nature. These proteins are not toxic, and their physiological role is unknown. The genes, cipA and cipB coding for the crystal proteins in Photorhabdus are known. The Xenorhabdus intracellular crystals are smaller and the genes coding for them are not related to those of Photorhabdus (see the review of Forst, 2002). Mutants or phase variants not producing intracellular crystals are neither producing antibiotics nor building symbiosis with entomopathogenic nematodes. As it will be discussed in Chapter 3 (Results) we have discovered an exo-cellular crystal produced by only one Xenorhabdus strain (EMC) isolated in our laboratory. This crystal is not protein, but a polysaccharide metal complex. Although we have crystalminus mutants, and have isolated the compound, we do not know anything about its biological role. 20 1.2. OBJECTIVES AND RATIONALE This study is aimed at revealing some details of the nature and evolution of the developmentally controlled, intimate, and highly taxon-specific symbiosis of entomopathogenic nematodes (EPN) and bacterium (EPB) species. The ultimate goal is benefiting from this information for improved biological pest control in the agriculture. I intended to provide examples of using selected EPN strain against target insect pests and EPB strains against target microbial pests and focus on the potential of producing new EPN/EPB symbiotic complexes. Biological control is characterized by the utilization of living microorganisms to control pests. Gnotobiology comprises a study of germ-free plants and animals to which specific microorganisms are coupled by experimental methods. When one or more known species of microorganisms are added experimentally to a germ-free animal, the host is no longer germ – free. Both the host and the introduced species are gnotobiological. Gnobiological research seeks to explore the effects of microorganisms in natural diseases, to identify the specific causative agents in infectious diseases. Gnotobiology is a part of the microbial ecology, which studies the relationships between animals and their associated microbial populations. An axenic animal is an animal living and reproducing free from any microorganism. Entomopathogenic nematodes are a welcome addition to the natural enemy pool of insects and can be integrated with various control measures for management of those target pests where individual tactics alone are inadequate. Entomopathogenic nematodes play a role underground reminiscent of that played by insect parasitoids. Like parasites or predators they have chemoreceptors and are motile. Like pathogens they are highly virulent, killing their hosts quickly and can be cultured easily in vivo or in vitro. Entomopathogenic nematodes are among the best known of an otherwise poorly studied group of natural soil insect enemies. Interest in these beneficial organisms has increased rapidly in recent years and research are being conducted in many laboratories world-wide (Gaugler et al, 1997). New species are described every year and many more isolates are waiting for identification and study (Koppenhofer and Kaya, 1999). 21 The aims of the study are: 1. Screening the EPB bank of our laboratory to determine the antibiotic production of different Xenorhabdus and Photorhabdus strains, to compare the effects of the antibiotics on closely related strains and on taxonomically unrelated bacteria, such as E. coli, Erwinia amylovora B. subtilis, and B. cereus. 2. To determine the symbiotic partner range of a large number of Steinernema and Xenorhabdus strains (gnotobiological analysis). 3. To identify new Steinernema isolates by using a molecular tool (GeneGel Excel kit), cross fertilization and gnotobiological studies. 4. To establish a liquid fermentation technique of a new Steinernema isolate. 22 2. MATERIALS AND METHODS 2.1. ENTOMOPATHOGENIC NEMATODE (EPN) AND BACTERIA (EPB) USED IN THIS STUDY 2.1.1. EPN STRAINS Table 1. Summarizes the Steinernema strains from our laboratory collection and their natural Xenorhabdus symbionts. Table 1. Steinernema strains and their natural symbionts. Steinernema species Strains Origin Xenorhabdus symbiont S. feltiae Filipjev Umea Russia Sweden X. bovienii X. bovienii SF22 Finland X. bovienii Vija Norway Norway X. bovienii Scp Poland X .bovienii 1048 Galyatetõ X. bovienii 1003 Hortobágy X. bovienii Nyíregyháza Nyíregyháza X. bovienii IS6 Israel X. bovienii Sulcatus USA X. bovienii Mexican Mexico X. nematophila T1 Azores X. nematophila T2 Azores X. nematophila SK98 Unknown X. nematophila NCI N. Caroline X. poinarii NCI LU N. Caroline X. poinarii NC 513 N. Caroline X. poinarii KMD 15 Ohio, USA (A. Lucskai) X. spp. S. anomali S. intermedia Anomali W. Europe X. spp. BIOSYS ? X. spp. S. rarum Rarum S. America X. spp. S. bicornutum Bicornutum Europe (B. Tallósi) X. spp. S. affinis Affinis ? X. spp. S. cubana Cubana Cuba X. spp. S. scapterisci Scapterisci S. America X. spp. S. riobrave Riobrave Brasilia X. spp. S. spp. SK 27 ? X. spp. S. kraussei Kraussei Alps X. spp. S. bibionis Bibionis USA X. spp. S. spp. 336 ? X. spp. S. spp. Mongolian Mongolia (H. Pamjav) X. spp. S. carpocapsae S. glaseri S. ohioensis 23 3.1.2. EPB STRAINS Table 2. Summarizes the Xenorhabdus strains in the gnotobiological study. Table 2. The Xenorhabdus strains. A. Known, B. unknown (newly identified) strains. XENORHABDUS X. bovienii DSMZ 4766 X. poinarii DSMZ 4768 X. beddingii DSMZ 4764 X. nematophila DSMZ 3370 X. nematophila ATTC 19061 2. X. nematophila ATTC 19061 1. X. nematophila ANT 1 X. nematophila AN6 2. X. nematophila AN6 1. X. nematophila 703 X. nematophila N2-4 SS (2.) X. nematophila N2-4 SA (2.) X. nematophila N2-4 P (1.) X. nematophila T1 Xenorhabdus spp. SK 98 X. scapterisci 1 X. scapterisci 2 X. bovienii SF22 X. bovienii Norwegian “Mongol” X. bovienii Sulcatus X. bovienii Filipjev X. bovienii Umea X. bovienii HU1003 X. bovienii IS6 X. bovienii Nyíregyháza X. spp. kraussei X. spp. „affinis” X. spp. „rarum” X. spp. „kushidai” X. bibionis X. spp. „serratum” X. spp. cubanum S47A X. riobrave 1. X. riobrave 2. KMD15 X. bicornutum X. intermedium X. intermedia BIOSYS X. anomali Lucskai X. anomali Azores A. SYMBIOTIC PARTNERS STEINERNEMA S. feltiae S. glaseri S. intermedium S. carpocapsae S. scapterisci S. feltiae S. kraussei S. affinis S .rarum S. kushidai S. bibionis S. serratum S. cubanum Steinernema sp. S. riobrave S. glaseri (strain Ohioensis) S. bicornutum S. intermedium S. anomali 24 Table 2. (continued) XENORHABDUS B. SYMBIOTIC PARTNERS STEINERNEMA Z 06 Z3 1/1 H5 FA 019 (Randy Gaugler) FA 013 49. HW 7 50. HK 2001 51. S 47A 52. KMD 44 Strain 3905 Unknown Steinernema from Transylvania Unknown Steinernema from Ohio (M. Klein) Unknown Steinernema, Washington State 2.2. METHODS AND TECHNIQUES 2.2.1. MICROBIOLOGICAL METHODS 2.2.1.1. SOLUTIONS AND MEDIA M9 a buffered physiological salt solution for nematodes (Brenner, 1974) KH2PO4---------------3 g Na2HPO4------------- 6 g NaCl-------------------5 g Fill it up with distilled H2O up to1000 ml After sterilization add: MgSO4 1 M------------1ml Liquid (or solid) LB media (Luria Bertani medium, Miller, 1972) (without or with agar): Bacto- trypton---------------- 10 g Bacto-yeast extract----------- 5 g NaCl-----------------------------10g Distilled H2O up to1000 ml (Agar: 15 g, if LB agar media is needed). Fill it up to 1 liter. Shake until the solutes have dissolved. Adjust the pH to 7.0 with 5N NaOH (0.2ml). Adjust the volume of the solution to 1 liter with deionized H2O. Sterilize by autoclaving for 20 minutes at 15 lb/sq. in. liquid cycle. Liquid (or solid) TSY media (without or with agar): Yeast extract----------------------5 g Soy peptone----------------------30g 25 Cholesterol------------------------3 ml/l Distilled H2O up to1000 ml (Agar: 15 g, if TSY agar media is needed). Liquid (or solid) NA media (without or with agar): Beef extract------------------------3 g Peptone-----------------------------5 g Sodium chloride-------------------5 g (Agar: 15 g, if NA agar media is needed). Fill it up with distilled H2O up to1000 ml NBTA an indicator medium Meat extract-----------3 g Pepton------------------5 g Agar--------------------15 g Fill it up with dH2O up to-1000 ml After sterilization and moderate cooling: BTK 25mg /ml---------1 ml /L (Bromothymol blue) TTC 40 mg /ml---------1 ml /L (Triphenyl tetrazoliumchloride) (An indicator media used to distinguish between primary and secondary forms of Xenorhabdus and Photorhabdus strains.) MacConkey Indicator Agar MacConkey Agar 47 g Bidistilled water 1 liter Expected results distinguishing between primary and secondary forms Media Primary Secondary MacConkey NBTA Tween Blood agar Brown Greenish Granules Hemolysis The colony is clear Red to Brown No granules No hemolysis Wouts” agar media Per liter Agar------------------------------12 g Bacto Peptone------------------10 g Beef extract----------------------6 g Vegetable or fish oil------------5 g Bidistilled water to 1000ml 26 2.2.1.2. METHODS ISOLATION OF THE SYMBIOTIC BACTERIA FROM DAUER JUVENILES The bacterium strains were isolated from cadavers of Galleria mellonella infected with Heterorhabditis spp or with Steinernema spp strains as described by Akhurst (1980). The protocol was modified as follows (A. Lucskai, unpublished): 1. The covering part of a sterile (Falcon) Petri plate was taken in a laminal box under a binocular microscope. 2. Add 200µl of 5% sodium-hypochlorite (NaOCl, chlorox). 3. Add 4X200 µl of sterile M9 buffer (Brenner, 1974) on the plate. 4. After washing the dauer with sterile tap water (1x) and M9 (3X) by centrifugation, transfer them into the drop of Sodium hypochlorite with the platinum wire and keep them there for five to seven minutes. 5. Transfer the dauers into the 1st drop of sterilized M9 buffer. Then transfer to the 2nd, and so on. 6. Transfer the nematodes into the last drop of sterilized M9 buffer by a platinum wire. 7. Cut the dauer into two or more parts. 8. Transfer about 2 µl of the suspension into LB or directly on LBTA indicator plates with a sterile automatic pipette. 9. The bacteria were grow on the plate at 30 °C. On indicator plates the primary form of the bacteria can be distinguished from the secondary forms and the contamination. (On the NBTA plate the color of the primary form colony is blue, all the others (contamination or secondary variants) are red. Bacterial strains were selected based on colony morphology on nutrient agar (Difco), ability to absorb dye when grown on (NBTA) medium and MacConkey Agar (DIFCO). Production of antibiotic substance was detected as described by Akhurst (1982). The secondary form lacks inclusion bodies as well as antibiotic activity as described by Boemare and Akhurst (1988). FREEZING OF BACTERIA FOR LONG-TERM STORAGE 1. One colony of any strain of bacteria was inoculated into sterilized liquid LB medium (5ml). 2. 1 ml suspension was placed into a sterile Eppendorf tube. 27 3. 0.5 ml glycerol (8.7%) was added. 4. Vortex for 30”. 5. Eppendorf tube was put into - 80 ˚C. 6. When taken out of the freezer 100 µl of the melt suspension was placed on LB agar plate. SURFACE STERILIZATION OF INFECTIVE DAUER JUVENILES 1. The dauer juveniles were washed 3 times with autoclaved tap water and centrifuged at low speed (~1,000 rpm) for 1 min. 2. The nematodes were surface sterilized in 0.05 % hyamine solution with vortexing every minute for 10 sec. 3. The IJs were centrifuged at 2,000 rpm for 1 min. and washed quickly with sterile M9 (hyamine kills the dauer stage when held too long). 4. The dauer juveniles were washed 3 times with sterile tap water. 5. Galleria mellonella (wax month) larvae were infected with the sterile nematodes. 2.2.2. METHODS FOR GNOTOBIOLOGY Some EPN species and strains were usually grown only on their own symbionts. Some of them can grow on each other symbionts in agar in Petri plates: Media TSY Woots NGM Bacterium X. bovienii Norwegian P. temperata ssp. temperata HSH1 X. bovienii Norwegian P. temperata ssp. temperata HSH1 E. coli OP50 Nematode Steinernema feltiae Heterorhabditis megidis NEW HSH1 All strains of S. feltiae All strains of H. megidis NWE C .elegans For all gnotobiological analysis the following procedure was used: 1. Liquid cultures of the primary form of Xenorhabdus or Photorhabdus were grown overnight aseptically in 5 ml of nutrient broth at 30 °C in a test tube (one tube per flask to be inoculated). The bacteria then were transferred to TSY or lipid agar plates and allowed to grow for 2-3 days at 25 °C. 2. Axenic J1 juveniles of Steinernema or Heterorhabditis strains were transferred to bacterial lawn on TSY or lipid agar plates. Next generation infective dauer juveniles were collected. 28 3. Infective dauer juveniles (IJ) of several Steinernema or Heterorhabditis strains were surface sterilized and put on the bacterial lawn of the bacteria under gnotobiological investigation. The plates were scored based on: a. EPN grew (+) or did not grow (-) in into fertile adults. b. In the progeny population IJ appeared (+) or did not (-) appear. c. The IJ proved (+) or did not prove to be pathogenic for insect hosts (4th instar larvae of Galleria mellonella). d. The new generation of IJs left (+) or did not leave (-) the insect cadaver on water traps. e. The bacteria isolated from the IJs could be identified as the “new” (+) or the original (-) symbiont. 29 3. RESULT AND DISCUSSION 3.1. ANTIBIOTICS PRODUCTION OF THE EPB STRAINS 3.1.1. ISOLATION EPB SYMBIONTS DIRECTLY FROM DAUER LARVAE OF STEINERNEMA SPECIES The technique of direct isolation EPB symbiont from a single dauer larva was originally elaborated by A. Lucskai. The method is considerably safer than isolating the symbionts from an infected insect, since the contamination inside the carcass can be eliminated. The method was further modified in our laboratory (Fig. 4.). Fig. 4. Isolation of EPB symbionts from EPN infective dauer juveniles LEGEND to Fig. 4. Isolation of the symbiotic EPB bacterium from surface sterilized IJ nematode. The dauer larvae obtained from water trap are washed with M9, bleach, and with M9 again. The nematodes were cut into pieces in sterile M9. Drops of the M9 solution containing the bacteria coming out from the gut of the IJ are transferred to indicator (NBTA) plates. Blue colonies are considered to be the primary form of the bacterial symbiont. 30 3.1.2. ANTIBIOTICS PRODUCTION OF DIFFERENT XENORHABDUS AND PHOTORHABDUS STRAINS TESTED ON BACILLUS CEREUS The antibiotics production of 103 strains was tested using Bacillus cereus as indicator bacteria based on Akhurst’s method (Akhurst, 1980). Figure 5. demonstrates how the effect of the antibiotics produced by EPN strains on B. cereus was determined. An EPB colony was grown (from a 10µl of overnight suspension) in the center of the LB agar plates. After 5 days the bacteria are killed in a chloroform fume and overlaid by the indicator bacteria (B. cereus spores) suspended in soft agar. The size of the inactivation ring is proportional to the antibiotics activity of the EPB strain studied. The antibiotic productions of different Xenorhabdus strains are variable. The results are presented in Table 3. Fig. 5. Antibiotics production of EMC (left) and X. bibionis (right) determined by using B. cereus as indicator strains. Inhibition zone LEGEND to Fig. 5. EPB bacteria were grown for 5 days in the center of the Petri dish and then the EPB was overlaid by the indicator strain resuspended in soft agar. The inhibition zone is shown as a ring around the EPB colonies. The strain EMC (left) produced excessive amount of antibiotics inhibiting the growth of the indicator strain almost completely. X. bibionis produced less antibiotics against B. subtilis, the inhibition zone is clearly visible on the plate. As it can be seen in Table 3. the best antibiotics producing strain was Ema with inhibition zones between 80-90 mm. The Ema strain (Ema new) was reisolated from the original nematode symbiont and the bactera were tested again for producing antibiotics. 31 The inhibition zone of the new Ema isolate was 87mm, and so there is a slight decrease compared to the original bacterial stock stored in the freezer. It is possible, that storing the nematodes in stock and allowing the reproduction “artificially” in G. mellonella does not help the bacterial strain to keep the antibiotics production (or other traits) at the same level and by time the laboratory conditions decrease or diminish the antibiotics production. The KMD15 and the DSM3370 X. nematophila strains also produced larger amount of antibiotics, the inhibition zones for B. cereus were 56 and 49 mm, respectively. The antibitotics production was tested with the colony on the plates and with the removed EPB colonies as well (Table 3.). In all cases the inhibition zones were larger (the antibiotics production was higher) when the colonies were present on the plates. The inhibitions zones were approximately one third smaller without the colonies. It is possible that the bacterial colony is releasing another – and not very stable - compound that makes the antibitotics more effective. Alternatively, if some of the produced antibiotics are membrane bound (the membranes of the bacteria are destroyed with the chloroform treatment) some of the activity will be lost as well. This way removing the colony means less “active” antibiotics in the media. When the fermentation soup was used, the inhibition zones were even smaller in each case. If the produced antibiotics and their activity are depending on a factor that is membrane bound, with fermentation of the bacteria, the inhibition zone would be smaller. Alternatively, liquid fermentation conditions are less preferable for the antibiotics production than the solid surface aerobic growth. As control for the EPB antibiotics production Ampcillin (100ppm) was used. 32 Table 3. Antibiotics production of the EPB strains against B. cereus. Petri dishes overlaid by B. cereus spore suspension in soft agar EPB strains 1. 2. 3. 4. 5. 6. 7. 8 9. 10. 11. 12. 13. 5-days old EPB colony Diameter of the inhibition zone (mm) 1. 2. Average X. Ema1 old 90 90 90 >80 X. Ema1 new 87 87 87 X. Kmd15 56 56 X. n. N2-4/I 25 X. n. N2-4/Ii 200 μl of fermentation soup Diameter of the inhibition zone (mm) Average 1. 2. Average >80 >80.0 30 30 30 77 77 77.0 30 30 30 56 46 46 46.0 17 17 17 23 24 12 9 10.5 20 20 20 14 12 13 3 2 2.5 13 13 13 DSM 3370 55 43 49 53 43 43.0 15 15 15 P. Jun old 29 28 28.5 20 19 19.50 12 12 12 P. Jun new 27 26 26.5 18 17 17.5 12 12 12 P. Eg2 24 24 24 15 14 14.5 14 14 14 P. Eg2 new 23 24 23.5 12 13 12.5 15 15 15 P. Arg 41 34 37.5 32 25 28.5 13 13 13 P. IS5 28 29 28.5 19 20 19.5 14 14 14 P. OH-I 13 14 13.5 3 4 3.5 16 16 16 28 23 26.5 Colony removed Ampicillin 100 ppm LEGENDS to Table 3. Comparison of the antibiotic production of several Xenorhabdus (X.) and Photorhabdus (P) isolates. There was no significant difference between old and new isolates of the same strain. Abbreviations: Ema: Xenorhabdus symbiont of S. bicornutum strain Tallósi; old: refrozen stock; new: freshly isolated from the nematode just for this experiment; P. Jun: Photorhabdus stackebrandtii; 33 isolated from Heterorhabditis megidis (NWE) strain H. Jun by Emília Szállás; and re-isolated by Attila Lucskai. X. Kmd15: Xenorhabdus sp. isolated from S. glaseri strain Kmd 15 (Ohio); discovered by Attila Lucskai, and the symbiont was also isolated and re-isolated by him; X.n.: X. nematophila (natural symbiont of S. carpocapsae). I: phase I (primary form); phase II (secondary form); Ii: an intermediate form. P.Eg2: P. luminescens ssp. akhurstii; isolated from S. indica strain EG2 (from Egypt) by Emília Szállás and reisolated by Attila Lucskai. P. arg: P. luminescens ssp. laumondii from H. bacteriophora strain Argentinensis Emília Szállás and re-isolated by Attila Lucskai. P. IS5: P. luminescens ssp. akhurstii; isolated from S. indica strain IS5 (originated from the Negev desert, Israel, got from Prof. Itamar Glazer) by Emília Szállás and re-isolated by Attila Lucskai. P. OHI: P. temperata (originated from the Mohican National Park, Ohio, USA, got from Prof. Michael G. Klein) by Emília Szállás and re-isolated by Attila Lucskai. The reference strains (DSM) are originated from the DSMZ collection, Braunschweig, Germany, provided by Prof. Erko Stackebrandt. 3.1.3. THE EFFECT OF ANTIBIOTIC PRODUCTION ON OTHER ENTOMOPATHOGENIC BACTERIA The Xenorhabdus strains producED antibiotics against Photorhabdus and Photorhabdus strains are also producing antibiotics against Xenorhabdus. A comparison of the different EPB strains is demonstrated in Fig 6. The antibiotics produced by one EPB strain might be also effective against other EPB strains. It was supposed that antimicrobial compounds including antibiotics and colicins have an important role in competition between EPB (and related EPN) strains in nature. The sensitivities of the different Photorhabdus strain to antibiotics produced by a Xenorhabdus strain differed from each other. Figure 6 and Figure 7 give examples that the antibiotics of the symbiont of S. kraussei (X. bibionis) and S. affinis could be effective against Photorhabdus strains. As it is shown on Figure 6 the K122 strain showed to be the least sensitive against the antibiotics produced by the Xenorhabdus symbionts of S. kraussei and S. affinis, the most antibiotics were produced by X. bibionis and against HL81 (Fig 6. and Fig. 7). As shown in Table 4, the antibiotics produced by the symbionts of S. scaptersi, S. bicornutum, S. rara and S. intermedia were the most effective against the symbionts of H. bacteriophora. The symbionts of S. affinis and S. kraussei produced effective antibiotics against most of the P. bacteriophora strains. The symbionts of S. feltiae and S. serratum did not produce antibiotics effective against the P. bacteriophora strains tested. It is also shown that the A1 and Koh strains were the most insensitive to the antibiotics produced by Xenorhabdus. Table 5 shows the antibiotics produced by the symbionts of S. scaptersi, S. bicornutum, S. rara and S. intermedia were the most effective against the symbionts of H. megidis, H. marelatus and the Wisconsin isolates. The symbionts of S. affinis produced effective, but less, antibiotics against most of the Photorhabdus symbionts. Again, the symbionts of S. feltiae and S. serratum did not produce antibiotics effective 34 against most of the Photorhabdus strains tested. It is also shown that the A1 and Koh strains were the most insensitive to the antibiotics produced by Xenorhabdus. From the data it might be concluded that in some cases the coexistance between the symbionts of Xenorhabdus and Photorhabdus is possible if there are no other products of the nematodes or the bacteria that would inhibit the growth. For instance, H. bacteriophora A1 strain could infect the same insects that S. affinis already infected. Based on our data, theoretically symbiotic bacteria of different nematode species could coexist in the same invironment allowing horizontal gene flow. Explain Table 4., table 5., table 6. Data also show that different Photorhabdus strains of H. bacteriophora symbionts belonging to the presented subspecies of the P. luminescens species show a similar degree of sensitivity to the different Xenorhabdus antibiotics. Interestingly, the toxin-producing P. luminescens ssp. akhurstii strains (symbionts of H. indica) are very sensitive to the Xenorhabdus antibiotics, as demonstrated in Table 5. Fig. 6. A qualitative and quantitative comparison of the antibiotics produced by different Xenorhabdus strains. LEGEND to Fig. 6. A comparison of affectivity of the antibiotics of different Xenorhabdus strains against different Photorhabdus strains. The symbiont of S. kraussei (X. bibionis) is in the middle and the indicator strains are A. K122, B. HP88, C. HL81. The symbiont of S. affinis is in the middle and the indicator strains are D. K122, E. Hb1, F. OH10. The most effective antibiotics were produced by X. bibionis against HL81strain among the strains tested in this experiment. 35 Fig. 7. X. bibionis strain inhibiting the growth of Photorhabdus temperata ssp. temperata strain HL81. Error! Inhibition zone LEGEND to Fig. 7. X. bibionis generally produced antibiotics with low effectivity against indicator bacteria (see Table 5. and Fig. 6), but exerts a stronger inhibiting activity against Photorhabdus strain HL81. 36 Table 4. Antagonism between Xenorhabdus and Photorhabdus. I as shown in inhibition zones (mm) Symbiont(s) of Steinernema scapterisci bicornutum rara intermedia affinis kraussei (X.bibionis) anomali Symbionts of H. bacteriophora strains P. luminescens subspecies luminescens laumondii boemarii Ind IS5 EG2 Hm1 Hb1 HP88 A1 Koh MOL 38 22 38 43 41 44 37 38 37 24 28 13 12 11 25 19 20 15 14 27 30 18 11 10 26 25 13 8 8 25 30 15 14 10 29 15 7 10 7 30 34 16 0 0 32 37 17 11 0 21 30 17 0 8 0 0 0 0 14 0 0 0 0 0 13 8 23 14 13 0 12 0 0 0 0 0 0 0 0 5 0 0 6 0 0 0 14 14 0 0 0 0 0 0 4 0 0 0 0 0 7 0 5 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 carpocapsae (X. nematophilus) DSM 3370 N2-4 0 15 glaseri (X. poinarii) DSM 4768 Kmd 15 Cubana 8 12 0 feltiae (X. bovianii) DSM 4766 Nyíregyháza 0 0 serratum (X.beddingi) DSM 4764 0 LEGENDS to Table 4. Antagonism between Xenorhabdus and Photorhabdus. The symbionts of Steinernema species (S. scapterisci, S. bicornatum, S. rara, S. intermedia, S. affinis, S. kraussei, S. anomali, S. carpocapsae, S. glaseri, S. feltiae and S. serratum) were grown for two days on TSY agar plates and the sensitivity of P. luminescens strains (the symbionts of H. bacteriophora) for the produced antibiotics was measured through the size of the inhibition zone. The data are representative, the experiments were repeated three times. 37 Table 5. Antagonism of Xenorhabdus vs. Photorhabdus. II. Photorhabdus symbionts of Symbiont of Steinernema scapterisci bicornutum rara intermedia affinis kraussei (X. bibionis) carpocapsae (X. nematophilus) DSM 3370 N2-4 glaseri (X. poinarii) N2-4 glaseri (X. poinarii) DSM 4768 Kmd 15 Cubana feltiae (X. bovianii) DSM 4766 Nyíregyháza serratum (X.beddingi) DSM 4764 H. megidis NWE (ssp.temperata) US H. marelatus Wisconsin isolates HSH2 31 HL81 49 H4 35 Meg1 47 OR10 41 Hepialius 42 Wx6 45 Wx8 50 14 35 21 10 0 35 49 28 0 27 20 28 14 7 0 38 36 0 12 0 32 17 20 10 5 35 28 19 13 0 38 42 18 8 8 29 41 19 12 0 0 0 0 27 18 17 0 0 0 0 0 0 5 10 0 0 0 0 8 0 0 0 0 0 0 37 7 0 0 0 0 0 0 11 0 14 20 0 4 0 0 0 0 0 5 0 0 0 0 0 8 0 0 0 0 0 0 0 0 14 0 0 0 0 0 0 0 0 0 0 0 0 0 0 7 0 0 0 0 0 LEGENDS to Table 5. Antagonism between Xenorhabdus and Photorhabdus III. The symbionts of Steinernema species (S. scapterisci, S. bicornatum, S. rara, S. intermedia, S. affinis, S. kraussei, S. anomali, S. carpocapsae, S. glaseri, S. feltiae and S. serratum) were grown for two days on TSY agar plates and the sensitivity of Photorhabdus strains (the symbionts of H. megidis, H. marelatus and the Wisconsin isolates) for the produced antibiotics was measured through the size of the inhibition zone. The data are representative, the experiments were repeated three times. 38 Table 6. Antagonism of Xenorhabdus vs. Photorhabdus. IV Photorhabdus symbionts of H. downesii Xenohabdus strain, symbiont of Steinernema scapterisci bicornutum rara intermedia affinis kraussei anomali carpocapsae DSM 3370 N2-4 glaseri DSM 4768 Kmd 15 Cubana feltiae X. bovianii DSM 4766 X. bovianii Nyíregyháza serratum Irish ssp. temperata Hungarian H. megidis P. stackebrandtii H. sapiens Wisconsin isolate P.asymbiotica P.nealsonii WX13 46 K122 45 HIT 42 Jun 41 ATTC 43951 13 33 30 17 10 0 0 36 15 17 10 0 0 25 11 15 7 4 0 0 15 14 9 12 0 37 39 12 9 12 24 0 0 0 0 0 0 0 16 16 0 0 0 0 0 0 0 7 0 0 0 10 10 9 15 12 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 LEGENDS to Table 6. Antagonism of Xenorhabdus vs. Photorhabdus IV. The data are representative, the experiments were repeated three times. Data concerning the effects of Xenorhabdus antibiotics on Photorhabdus strains belonging to the species Xenorhabdus antibiotics to Photorhabdus strains belonging to P. stackebrandtii, P. asymbiotica and P. nealsonii species. K122 strain belongs to P. temperata ssp. temperata and a symbiont of a strain (K122) of H. downesii. HIT strain, on the other hand, belongs to P. stackebrandtii and is a symbiont of another strain (HIT) of H. downesii. There is not too much difference between the sensitivity to Xenorhabdus antibiotics of the two, taxonomically different Photorhabdus strains. The most interesting data are those of P. nealsonii (WX13) and the human pathogen P. asymbiotica strain. The latter is the less sensitive the earlier is the most sensitive Photorhabdus strain we examined. 3.1.4. EFFECTS OF THE XENORHABDUS ANTIBIOTICS ON ERWINIA AMYLOVORA 3.1.4.1. EFFORTS TO CONTROL THE FIRE BLIGHT DISEASE IN APPLE ORCHARDS Fire blight is a destructive bacterial disease of apples and pears that kills blossoms, shoots, limbs, and, sometimes, entire trees. The disease is generally common throughout the mid-Atlantic region of the US and in Europe. The outbreaks are typically very erratic, causing severe losses in some orchards in some years, and little or no 39 significant damage in others. This erratic occurrence is attributed to differences in the availability of overwintering inoculum, the specific requirements governing infection, variations in specific local weather conditions, and the stage of development of the cultivars available. The destructive potential and sporadic nature of fire blight, along with the fact that epidemics often develop in several different phases, make this disease difficult and costly to control. Fig. 8. Fire blight disease in apple orchads. Overwintering cankers harboring the fire blight pathogen are often clearly visible on trunks and large limbs as slightly to deeply depressed areas of discolored bark, which are sometimes cracked about the margins. The largest number of cankers, however, are much smaller and not so easily distinguished. These occur on small limbs where blossom or shoot infections occurred the previous year and often around cuts made to remove blighted limbs (P.W. Steiner, University of Maryland, T. van der Zwet, USDA and A. R. Biggs, West Virginia University). Strains of the pathogen that are resistant to streptomycin are present in some orchards in the eastern USA and in Europe., and are widespread in most apple and pear regions of the western U.S. Biological control agents, although not widely used, have provided partial control of blossom infections. More effective biological agents are required if their use is to become widespread. 3.1.4.2. LABORATORY TESTS ON ERWINIA AMYLOVORA The majority of the tests were carried out in the Quarantine Laboratory of the Szent István University of Horticultural Sciences in Budapest, Hungary within a framework of co-operation. Some of the experiments were performed by M. Hevesi and S. Pekár and their data with their permission, are also incorporated in Table 7. Different Erwinia isolates were used as “indicator” bacteria to test different Xenorhabdus and 40 Photorhabdus strains for antibiotics production. The results were very reproducible. The results of the Erwinia laboratory tests are summarized in Table 7. Table 7. The effect of EPB antibiotics on ten E. amylovora isolates. Erwinia isolates: EPB strain Ea2 Ea60 Ea67 Ea9 Ea26 Ea56 Ea78 Ea47 Ea17 Ea28 E. coli K12 X. Ema1 X. Kmd15 X. N2-4-P X. N2-4Ii 26.6 6.0 28.5 23.0 25.5 6.0 28.5 23.0 23.0 5.5 27.0 24.0 28.0 6.0 28.0 23.5 26.5 3.0 23.0 22.5 22.5 2.0 25.5 24.5 21.5 4.0 25.0 24.5 24.0 2.0 25.5 24.5 27.5 3.0 24.5 22.5 24.5 1.0 22.0 21.0 25.5 3.8 22.7 23.3 X. N2-4/II X. DSM3370 P. Hit P. Jun P. K122 P. Az29 P. Eg2 P. OH-10 P. Arg P. IS5 P. Brecon P. HE86 P. indica P. Wx6 P. Az36 P. HP88 E. coli K12 26.5 28.0 18.0 19.0 26.5. 21.0 19.0 6.5 18.5 28.0 21.5 10.0 20.0 14.0 26.5 25.5 0 26.5 26.0 21.0 20.0 28.5 28.5 21.0 8.0 18.0 30.0 19.0 12.0 18.5 15.0 35.0 24.0 0 26.0 30.0 25.0 18.0 26.0 25.5 22.5 9.0 20.5 25.0 22.0 15.0 26.5 16.5 30.0 22.0 0 22.0 25.0 24.0 19.0 27.0 20.5 23.0 8.5 17.0 27.5 21.5 12.0 24.0 15.0 26.5 22.0 0 22.5 20.0 22.0 15.5 12.5 16.0 20.0 6.0 10.0 25.0 0 10.0 15.0 11.0 17.0 23.0 0 20.0 19.5 25.0 10.5 13.5 16.0 20.0 8.0 10.0 25.0 24.5 8.0 14.5 9.0 15.0 18.0 0 20.0 19.0 23.5 13.0 12.0 18.0 19.5 8.0 10.0 23.5 25.0 9.0 13.0 8.5 22.0 25.0 0 20.0 22.0 28.0 14.2 12.5 19.0 23.5 7.0 14.0 26.0 22.5 6.0 18.0 11.0 23.5 19.0 0 20.0 22.0 28.0 13.5 14.0 19.0 19.0 6.0 15.0 22.5 19.0 5.0 18.5 13.0 21.0 30.0 0 17.0 20.0 23.0 11.5 12.5 14.5 17.5 5.0 14.0 29.0 18.0 6.0 13.0 11.0 19.0 30.0 0 22.0 23.1 23.7 15.4 16.1 19.8 20.5 7.2 14.7 24.1 21.4 9.3 18.1 12.4 23.5 23.8 0 LEGENDS to Table.7. The symbionts of Steinernema and Heterorhabditis species were grown for two days on TSY agar plates and the sensitivity of the Erwinia isolates (Ea2, Ea60, Ea67, Ea9, Ea26, Ea56, Ea78, Ea47, Ea17 and Ea28) for the produced antibiotics was measured by the size of the inhibition zone. The data are representative, all experiments were repeated three times. The inhibition zones with the size above or equal to 30 are labeled with blue, and with green if the inhibition zone was above or equal to 24. E. coli strain K12 was used as control. The best antibiotics producing strains were among Photorhabdus strains (IS5, Az36 and HP88, labeled by blue), but the effectiveness was specific to certain Erwinia isolates. Xenorhabdus strains Ema1 and N2-4 produced antibiotics widely usable for most of the tested Erwinia isolates (green). Generally, all EPB strains produced antibiotics against the Erwinia isolates. The best results (antibiotic activity equivalent to 100-200 ppm streptomycin sulfate) were produced by the Xenorhabdus strains EMA and N2-4, and the Photorhabdus strain IS5 and Az36. Other EPB strains proved to be also very effective against the Erwinia isolates. In other tests (data are not shown) antibiotics produced by 20 EPB strains were also effective against Agrobacterium, Clavibacter, Pseudomonas, and Xanthomonas species (M. Hevesi, personal communication). The Erwinia isolates were collected from different plants and geographical locations by M. Hevesi. In larger scale EPB antibiotics were not tested so far against any plant diseases directly. Our results clearly indicate that the application of the EPB antibiotics might be a 41 very important tool for fighting the fire blight disease and presents a novel tool for fighting other bacterial parasites as well. In our laboratory the first attempt was made to investigate the antibiotics production of several strains of Xenorhabdus and Photorhabdus against Erwinia, and the data are promising for the development os commercially useful strains, or even mixture of strains, that kills Erwinia. 42 3.2. FERMENTATIVE PRODUCTION, CHEMICAL IDENTIFICATION AND APPLICATION OF EMA ANTIBIOTICS 3.2.1. FERMENTATION OF THE EMA STRAIN AND TESTING THE ANTIMICROBIAL ACTIVITY OF THE FERMENTATION SOUP We have optimized the conditions for growing several Xenorhabdus strains in liquid culture. We used both shake cultures, a small fermentor (G. Furgani, F. Főglein and A. Fodor) and a bioreactor (A. Szentirmai). In the bioreactor we grew the bacteria using 10 liters of volume in a 35-liter fermentor in Debrecen (Hungary). The products of fermentation (fermentation cell free supernatant) were directly tested for antibiotics production in the laboratory and phytotrone. The fractions of the fermentation soup were used to isolate and identify the biologically active compounds. The results of the tests are summarized in Figure 9. The figure shows that the antimicrobial activity of EMA (X. bicornutum) is extremely effective, the Erwinia test bactera were almost completely killed. In the phytotrone test the EMA strain produced antibiotics were as effective as kasumycin or streptomycin when the infection and the treatment were performed at the same time. Antibiotics were more effective when the plants were treated 24-48 h before the artificial infection. Even in diluted forms the fermentation soup proved to be very effective against Erwinia, although with dilution the efficiency decreased, the examined length of the infected area increased. The project was completed in cooperation with M. Hevesi. The results of the agar assays and the phytotrone tests are promising for fighting the fire blight in the apple and pear plantations and raise questions about the chemistry and biology of the active compounds. 43 Fig. 9. EMA antibiotics tests on E. amylovora on Petri dishes, liquid culture and phytotrone. Inhibition zone LEGENDS TO Fig.9. The antimicrobial activity of the fermentation soup of EMA (isolate 262). A: Antimicrobial activity of Xenorhabdus sp. EMA strain on E. amylovora agar plate. B: Phytotrone test of EMA fermentation soup. The biological activity of the EMA antibiotics can be compared to those of streptomycin and kasumycin. With the treatment of kasumycin the length of the infected area is greatly decreased. The fermentation soup (FS) of EMA is effective against Erwinia and the length of the infected area is decreased in the case of application of the FS in diluted (1:1 and 1:10) and undiluted form. With dilution of FS the infection increases. The experiment was comleted in duplicates twice in July (18. and 31.). 3.2.2. ISOLATION OF COMPOUNDS OF ANTIMICROBIAL ACTIVITY FROM FERMENTOR LIQUID CULTURE OF XENORHABDUS SP. EMA STRAIN Recently elaborated techniques have been identified for isolating active compounds from the fermentation soup of Xenorhabdus cultures. The experiments were carried out in co-operation, within the framework of OTKA grant T 035010 (B. Sztaricskai, Batta, M. Szentirmai, A. Fodor, G. Furgani, 2003). The strains were chosen for the study based on their ablility to produce antibiotics on Patri dishes using B. subtilis as indicator bacterium. The active compounds could be adsorbed to charcoal from the supernatant of the liquid culture or from the suspension of broken cells and eluted by a mixture of HCl and methanol (Fig. 10A.). The diluted compounds could be fractionated by paper chromatography. The biological activity was tested on B. subtilis. Both the supernatant and the cell fraction contained biologically active fractions. Comparing the activities to each other, with 11 mg of the charcoal separated EMA (isolate 262) supernatant fraction the inhibition zone was 12 mm, while with 200 mg of the active cell fraction the inhibition zone was 16 mm. With linear regression for 200 mg supernatant fraction the inhibition zone would be 218 mm, so it might be concluded that the supernatant fraction is biologically more active. 44 Two different isolates of the EMA strain (262 and 282) were tested for their biological activity against B. subtilis after the charcoal separation process. Table 8. shows that with fermenting of the isolate 262 the antibiotic activity was better, since with 1100 g/0.1 ml active supernatant compound the inhibition zone average was 18.5 mm, while the isolate 282 at 2000 g/0.1 ml active supernatant compound concentration the inhibition zone average was 20.5 mm. With linear regression the isolate 282 would have produced an 11 mm inhibition zone if the concentration of the active compounds was 1100 g/0.1 ml. It is also possible that the isolate 262 active compounds separated with charcoal process are more stable than that of the isolate 282. The stability of the water solution of the charcoal isolated EMA antibiotics was also tested for two different isolates of the EMA strain (262 and 282). Table 8. The stability of the water solution of the charcoal isolated EMA antibiotics at 4 Co after 6 months storage. Sample EMA Cc Isolate 262 1100 g/0.1 ml Isolate 282 2000 g/0.1 ml Streptomycin 50 g/0.1 ml B. subtilis Inactivation zone (mm) Right after isolation 6 month later 19 17 18 16 21 16 20 16 23 19 23 21 LEGENDS to Table 8. Comparison of the antimicrobial compounds of the same samples before and after storage in refrigerator. The antibiotics activity of EMA compounds is stabile, with 6 months of refrigation the inactivation zone caused fraction 1. (fermentation soup water soluble compounds) only decreased slightly. The experiments were performed in duplicates. 1. The material obtained from cell free supernatant. Several ninhidrin-positive fractions could be separated by chromatography, but only one fraction (Compound 1, comprising about 3.2%) proved to be biologically active against B. subtilis. 2. The biologically active compound could be also obtained from the cell mass, separated by chromatography to several ninhidrin-positive fractions, and one of them (Compound 2, comprising about a 2.6 %) proved to be biologically active, but its activity was significantly lower than that of the one originated from the cell-free supernatant. The two biologically active compounds were chemically very different. No synergism could be detected. The biological activity of Compound 1 was severely pH-dependent. Compound I was further purified by Kieselgel 60 [CHCl3-MeOH-NH4OH (8:2:0.25)] 45 column chromatography. We finally found a ninhidrin-positive, UV-active compound of low biological activity. By using 1H-, and13CNMR-, as well as mass spectrum (EI) analysis it was identified as Triptamin. 3. The rest of the biologically active compounds of unknown chemical structure were isolated by using an alcohol: hydrochloride elution. Both the pellet (Compound 2 about a 0.40 g) and the freeze-dried pellet (Compound 3, about 1.34 g) are active. The data on biological activity of the compounds isolated by the charcoal and Dovex50 method on B. subtilis are presented in Fig. 10 A and 10 B, respectively. The purified material obtained by the Dowex technique was hydrolyzed with 6 n HCl (105oC, 24 hrs). The identification of the 9 peptide fragments is in progress. Considering the presence of Triptamin amongst the active compounds we supposed it was nematophin (Webster et al, Patent number: 5,569,668. USA Patent, 27 October, 1996). Therefore we have synthesized nematophin in the following way: racem Triptamin was vacillated by 2-oxo-3-metil-penthane-carbonyl-acid. Briefly, the structure of the isolated Triptamin was proved by 1H and 13C NMR: the presence of four aromatic proton; three quaternary C; one vinyl-proton and two – CH2-- group. This compound was treated with 2-oxo-3-metil-penthane-carbonyl-acid in the presence of sulphonylic acid (SOCl2). In the presence of absolute pyridine 1 molecule of HCl was lost, and the product of the synthesis was the nematophin (D, L 3-indolethyl-3’-methyl-2’-oxo-pentanamid). The identity of the molecule was proven by E+MS, the molecular peak was 160 m/z. 46 Fig. 10A: Fractionation of the biologically active compounds adsorbed to charcoal. LEGENDS to Fig 10A. Concentration of the biological active compounds by using charcoal adsorption followed by fractionation. The supernatant of the fermentation soup was separated from the cells by centrifugation. The supernatant was incubated with Cellit to remove the lipids from the supertanatnt and filtrated. The filtrate was incubated with charcoal to bind the active compounds and the bound fraction was separated from the inactive suparnatant with centrifugation. The charcoal-active compound was washed with methanol and then the active compound was eulted from the charcoal with methanol/1N HCl (9:1). After neutralization with ammonium hydroxide the active compound was freeze-dryed.With processing 500 ml cell-free supernatant 0.53 g active crude product could be gained. After centrifuging 500 ml fermentation product, 63 g of cells could be processed. The incubation with 96 % ethanol/2N HCl (9:1) and centrifugation removes almost all the cell debris and leaves the active compound in the liquid. With freezedrying the weight of the active compound was 0.49 g. The biological activity of the two fractions was compared using B. subtilis as indicator bacteria and was compared to that of the Streptomycin. 47 Fig. 10B. Fractionation of the biologically active compounds adsorbed to DOWEX 50. LEGENDS to Fig 10B. Concentration of the biological active compounds by using charcoal adsorption followed by fractionation. 500 ml fermentation soup supernatant was incubated with pretreated resin and after filtration the inactive filtrate was removed. The compound was bound to Dowex50 and gradually eluted with 2-50 % methanol. The fractions were tested for biological activity. The active fractions were incubated with 80 % methanol and freeze-dried. The pellet was further purified with the incubation of 80 % methanol/1N HCl (99:1), neutralization and filtration and the filtrate was also biologically active. After freeze-drying the same product kept the biological activity. Biologically the most active fraction was the second with 24 mm inhibition zones compared to that of the first (12 mm) and third (18 mm) fractions. 48 Table 9. Comparison of the biological activities of the compounds with antimicrobial activity on B. subtilis. Compounds of antimicrobial activity Charcoal method Fermentation soup Compound 1 Compound 2 DOWEX50 method Compound 1 (Triptamin) Compound 2 Compound 3 Streptomycin Amount Origin Isolated by B. subtilis Inactivation zone (mm) 0.1 ml 11 200 Supernatant Supernatant Cells Charcoal 96% ethanol: 2n HCl (9:1) 15 12 16 0.5 Supernatant 80% methanol 12 0.5 0.5 50 Supernatant Supernatant - 80% methanol: 1n HCl (9:1) - 24 18 20 Table 9. summarizes the biological acticities of the fractions from the fermentation soup. The greatest biological activity belonged to Compound 2 isolated with the DOWEX50 method. The DOWEX50 method is more efficient, than the charcoal method. The final product (crude product) active ingredients give 12 mm inhibition zone for the indicator bacteria when using 11 mg of the product, while with DOWEX50 method Compound 3 gives 18 mm inhibition zone, when using 0.5 mg of the product. Since storing the freeze-dried product is the best way of keeping the activity for longer periods, the final active (crude) products should be compared in the freeze-dried form. The active compounds isolated with the DOWEX50 method is 36 times more efficient against the indicator bacteria than the ones isolated with the charcoal method. 49 3.3. THE EMC STRAIN The EMC strain was isolated from an S. rarum strain by E. Szállás (Eötvös University, Dept. of Genetics, Budapest, Hungary). Unusual phenotypic characteristics make this strain different from other Xenorhabdus strains: swarming behavior, color of the colony and the unique crystal production. 3.3.1. THE PHENOTYPIC CHARACTERIZATION OF THE EMC STRAIN 3.3.1.1. COLONY MORPHOLOGY AND SWARMING BEHAVIOR On indicator plates (NBTA and MacConkey agar) EMC exhibits typical Xenorhabdus phenotypes. On conventional media such as LB or TSY the EMC colony is brown, and the cells are pigmented within the colony. This phenotypic character is unusual for Xenorhabdus, in the EPB families only the Photorhabdus strains produce pigments. EMC exhibits an extreme swarming phenotype as well. Swarming behaviour is different from swimming, since swarming motility is very specific to solid agar surfaces and supposedly the flagella that are produced and responsible for swarming are different from those of swimming. The characteristic color of an EMC colony, and the swarming activity of the cells are shown in Fig. 11. Fig.11. Extreme swarming phenotype and color of EMC. 50 3.3.2. EXO-CRYSTAL PRODUCTION This is the first study to describe the spectacular and unique exo-crystals that appear in the medium and on the surface of the EMC colonies. The exo-crystals especially appear on rich solid media (Fig. 12. and 13). In older agar media, the colored exo-crystals appear on the surface of the colony as well (Fig. 12). Surprisingly, a different Xenorhabdus (EMD) isolate from another S. rarum strain does not produce exo-crystals and the color of the colony - similar to other Xenorhabdus strains - is blue on NBTA agar media (Fig. 13). Re-isolating the crystal-producing EMC strain from the original S. rarum was successful. Fig. 12. The metallic-like crystals appear not only in the media but also on the surface of older colonies in agar plates. LEGEND TO Fig. 12 The Xenorhabdus sp. EMC strain produces exotic crystals of unknown function and chemical character. In older cultures (especially in sugar-rich media) the crystals could be detected not only in the agar but also on the surface of the colonies. 51 Fig. 13. The exo-crystal producing EMC and no exo-crystal producing EMD. EMC EMD LEGEND TO Fig. 13. Exo-crystals produced by Xenorhabdus sp. EMC (left): not characteristic to other isolates (EMD, right) isolated from S. rarum. On NBTA the colonies of EMC and EMD are blue, the EMC crystals exhibit red color. The exo-crystals can be found in older liquid cultures and even in minimum media, if the carbon sources are adonitol or maltose (A. Máthé, G. Furgani, A. Fodor, 2003, unpublished). In the API tests the minimum media is supplemented with one single carbon source. With API 50 tests EMC was shown to be able to metabolize trehalose and N-acetyl-glucose-amine and under these conditions the EMC strain produced only small amount of crystal. EMC metabolizes D-glucose, D-fructose, D-ribose, sorbitol, inositol, glycerol, gluconate and 5-aceto-gluconate, but with the compounds as an exclusive carbon sources it does not produce exo-crystals. EMC does not metabolize lactose, Lxylose and methyl-β-xylopyranoside. The crystals seen in the API 50 “pockets” are demonstrated on Fig 14. 52 Fig. 14 A. Exo-crystal production of EMC in liquid minimum media. growth L-xylose crystals Adonitol No growth Methyl-βxylopyranoside LEGENDS to Fig. 14 A. If the carbon source is L-xylose, EMC can grow, but does not produce exocrystals. If adonitol is used, both growth and exo-crystal production is detectable. In methyl-βxylopyranoside supplemented minimal medium, EMC can not grow, the carbon source can not be metabolized. Since the exo-crystals are completely insoluble in water or ethanol, it was difficult to understand how the bacteria could secrete them into the medium. One plausible explanation might be that a water-soluble monomer is released, which forms a polymer in the media. The polymer is colored, making the colonies brown. 53 Fig. 14 B. Crystal production of EMC in liquid minimum media. D-cellobiose Maltose Lactose LEGENDS to Fig 14B. If the carbon source is D-cellobiose, EMC can grow, and produces some exocrystals. If maltose is used, both growth and exo-crystal production is detectable. In lactose supplemented minimal medium, EMC can not grow, the carbon source can not be metabolized. The exo-crystals isolated from the media could be observed by light microscopy (Fig. 15 A, B and C) and scanning electron microscopy (Fig. 16). The crystals can be identified by their specific shape and brownish red color, the later might make the colonies appear brown. The exo-crystals can be dissolved by chloroform or DMSO. The shape of the crystals depends on the C-source. The data of image (ThermoNoran) analysis is also presented (Fig. 16). The exo-crystal does not contain nitrogen, but contains carbon, 54 oxygen and boran. We propose that the chemical nature of the exo-crystal is a large molecular weight carbohydrate – boran complex. Fig. 15A. Light microscopic picture of the isolated exo-crystal (125X magnification). LEGEND to Fig 15 A. Magnification: 125X; Jenaval Light Microscope. Fig. 15B. Light microscopic picture of the isolated crystal (250X magnification). LEGEND to Fig 15 B. Magnification: 250X; Jenaval Light Microscope. 55 Fig. 15C. Light microscopic picture of the isolated crystal (1000X magnification). LEGEND TO Fig. 15 C. The exo-crystal in 1,000X magnification (Jenaval light microscope). Fig. 16. EM picture and image analysis of the exo-crystal. LEGEND TO Fig 16. EM picture (right) and its (ThermoNoran) image analysis (left). The exo-crystal does not contain N, but contains C, O and B. Supposedly, the chemical nature of the exo-crystal is a large molecular weight carbohydrate – boran complex. 56 3.3.3. ANTIBIOTICS PRODUCTION OF EMC EMC is one of the best antibiotics producing strains tested in our laboratory. The compounds of the fermentation soup exert cytotoxic activity reproducibly on both E. coli and E. amylovora (Fig. 17). The effect of the antibiotics is cytotoxic and not cytostatic. The antimicrobial compounds of Xenorhabdus from fermentative productions effectively reduced the number of Erwinia cells in liquid media, and within 60 minutes no living Erwinia cell could be counted. Fig. 17. The cytotoxic activity of EMC and EMA. LEGEND to Fig. 17. Comparison of the cytotoxic (bactericide) activities of the 1:1 dilutions of the fermentation soup of EMC (blue) and EMA (pink) against E. amylovora. The EMA strain is an isoltate living in symbiosis with S. rarum as well. The antimicrobial compounds of both strains effectively reduced the number of Erwinia cells in liquid media and within 60 minutes no living Erwinia cell could be counted. Abscissa: the time in minutes; ordinate: the number of E. amylovora cells. (The exponentials of 100, 102, 104, 106, 108, 1010 are labelled as 1,00+00, 1,00+02, 1,00+04, 1,00+06, 1,00+08 and 1,00+10, respectively). EMC antibiotics also inhibit the growth of Phytophtora and Trichoderma species (Fig. 18). The tests were carried out in cooperation with Prof. Tibor Érsek (Institute of Plant Protection, Hungarian Academy of Sciences). The results indicate that the isolated antibiotics from EMC fermentation soup (DOWEX50 method) inhibit the colony forming of Phytophtora sp. With the application of 12.5 ppm antibiotics some growth still can be detected, but the concentration of 25 ppm inhibits the growth of Phytophtora completely. 57 Fig. 18. Effect of EMC antibiotics on the colony formation of Phytophtora sp. LEGEND to Fig 18. The antibiotics isolated from EMC fermentation soup (DOWEX50 method) inhibit the colony forming of Phytophtora sp. The concentrations are given in ppm. 3.3.4. CONCLUSIONS EMC is one of the most interesting strains of our stock collection. The swarming behavior as well as the color of the colony is unusual for Xenorhabdus. The crystal production is unique. The biological role of this carbohydrate – polymer metal complex (Furgani et al., in preparation) is unknown. The data from API tests confirm that the crystals are synthesized from sugars, which might be metabolized by EMC when the carbon sources are depleted. The genes that are responsible for the production of exocrystals are unknown. Screening 7,000 Tn10 transposon-induced EMC colonies we have generated 10 mutants, which do not produce exocrystals. None of the (10) mutants lacked the antibiotic production. Thus the genes that regulates the production of exo-crystals and the antimicrobial compounds may be independently regulated. The genomic regions where the transposon inserted are yet to be cloned and sequenced. 58 3.4. GNOTOBIOLOGY 3.4.1. RESULTS IN GNOTOBIOLOGY The Steinernema strains are capable of reproducing and infecting/killing caterpillars while carrying EPB symbionts of other Steinernema strains. In our laboratory Katalin Lengyel and Erzsébet Böszörményi (PhD students) performed gnotobiological analysis of Heterorhabditis /Photorhabdus complexes as well. The complete gnotobiological analysis of S. carpocapsae and S. feltiae isolates - with their permission is presented in Table 13-16. Table 10. Summary of the gnotobiological tests of Heterorhabditis / Photorhabdus complexes (with kind permission of E. Böszörményi and K. Lengyel). Bacteria EPB Hepialiu s H4 OHI Jun HSH2 K122 HIT Brecon HP88 A1 Az29 IS5 Wx4 Wx6 Wx11 Nematodes EPN H. marelata natural host H. mareliata Hepialius +++ H4 + H. megidis OHI + Jun + K122 - H. downesi HIT +++ H. bacteriophora A1 +++ H. megidis unknown unknown unknown H.downesi unknown H. bacteriophora unknown unknown unknown H.indica unknown unknown unknown +* +++ +++ +++ +++ +++ +++ +++ + +++ + + +++ + + +++ + + +++ + +++ +++ +++ +++ nt +++ nt +++ + + +++ +++ +++ + ++ ++ ++ ++ ++ ++ +++ +++ +++ +++ +++ +++ +++ + +++ +++ +++ +++ + + + nt nt +++ + nt nt nt nt nt +++ nt + + + +++ +++ +++ +++ +++ +++ ++ +++ +++ LEGEND to Table 10. -: not growing at all; +: J1 develops to fertile adult; ++: the new EPB symbiont is retained and was reisolated after several generations; nt: not tested; +*: J1 could not be recovered, but the IJs developed into fertile adults. Table 10. summarizes the gnotobiological analysis of the Heterorhabditis / Photorhabdus complexes. The tests show that H. marelata and H. bacteriophora can develop into fertile adults and IJs on almost all Photorhabus strains tested, but the nematode strains belonging H. megidis and H. downesi are not as successful, although three and two strains were tested, respectively. 59 Table 11. shows how the strains of S. carpocapsae (Mexicana and T1) and S. feltiae (SF22 and IS6) can develop on several strains of X bovienii (S. feltiae symbionts) and on the closely related X. affine, X. bibionis and X. kraussei. Most of the nematodes from both species developed into fertile adults, but the non-natural symbion could not be reisolated after several generations. The most successful nematode was the strain IS6, which could develop into fertile adults on almost all non-natural symbiont and the bacteria could be reisolated after several generations of growth. Table 11. Growth of S. carpocapsae and S. feltiae strains on the symbionts of S. feltiae and closely related species. Steinernema carpocapsae Mexicana T1 S. feltiae symbionts X. bovienii Nyíregyháza X. bovienii DSMZ4766 X. bovienii 1003 X. bovienii 1S6 X. bovienii Umea X. bovieni Finnish X. bovieni Norwegian X. bovieni Mongolian Closely related species X. affine X. bibionis X. kraussei Steinernema feltiae SF22 IS6 + + + + + + + + + +++ ++ + ++ ++ + ++ + + +++ ++ + + + ++ +++ +++ +++ +++ +++ +++ - + + + + + + + ++ +++ + +++ LEGENDS to Table 11. Practically all S. feltiae strains (but not the S. carpocapsae strains) can grow, propagate and produce infective dauer juveniles on the other Steinernema symbiont. Thy can also grow on the symbiont of S. kraussei and S. affine. -: not growing at all; +: J1 develops to fertile adult; ++: the new EPB symbiont is retained and was reisolated after several generations; +++: even more nematodes were produced, than at ++. The data in Table 12. records the same nematode strains as Table 11., but with the isolates of X. nematophila symbionts. The symbiotic relationship of the same nematodes to X. nemtophila looks less efficient, less strains support the growth of S. feltiae and even the strains of S. carpocapsea were not retaining the bacterial strains form different isolates of X. nematophila. Interestingly, although ATCC19061 primary strain supported the growth of S. feltiea Mexicana strain, N2-4 secondary and X. riobrave secondary strains supported the growth while their primary pair did not. Table 13. shows how the nematode strains of S. carpocapsea and S. feltiae can develop and reproduce on the symbionts of Steinernema species with short dauer phenotypes. The results clearly indicate that the nematodes only sporadically develop 60 into fertile adults retaining the bacteria. S. feltiae Mexicana strain is not able to retain these bacteria at all after several generation. Table 14. contains the data of the nematodes developing on symbiotic bacteria with unknown origin. The bacteria seem to support the nematode development better than the previously described species (Table 13.), and most of them provide good conditions for the S. feltiae Mexicana strain. Table 12. Growth of S. carpocapsae and S. feltiae strains on the symbionts of S. carpocapsae and related species. Steinernema carpocapsae Mexicana T1 S. carpocapsae symbionts X.nematophila DSMZ3370 X.nematophila T1 X.nematophila ATTC19061/2 X.nematophila ATTC19061/1 X. nematophila ANT 1 X.nematophila AN6 2 X.nematophila AN6 1 X.nematophila 703 X.nematophila N2-4 SS (2) X. nematophila N2-4 SA (2) X. nematophila N2-4P (1) S. riobrave symbionts X. riobravis/1 X. riobrave/2 S. kushidai symbionts X.kushidai Steinernema feltiae SF 22 Mexicana + + + + + + + + ++ +++ + + ++ + ++ + + + +++ + + + - +++ + - + + +++ ++ + + - +++ - - LEGENDS to table 12. Almost all S. carpocapsae strains (but not the propagate and produce infective dauer juveniles on other symbionts. Xenorhabdus symbiont of S. riobrave but not that of and S. kushidai. develops to fertile adult; ++: the new EPB symbiont is retained and generations; +++: even more nematodes were produced, than at ++. S. feltiae strains) can grow, Thy can also grow on the -: not growing at all; +: J1 was reisolated after several 61 Table 13. Growth of S. carpocapsae and S. feltiae strains on Xenorhabdus symbionts of other Steinernema species of short dauer phenotypes. Steinernema carpocapsae Mexicana T1 Steinernema feltiae SF 22 Mexicana Symbionts of Steinernema species with short dauer phenotypes S. rarum symbionts X. rara S. scapterisci symbionts X. scapterisci/1 X. scapterisci/2 S. bicornutum symbionts X. bicornutom S. serratum symbionts X. serratum S. anomali symbionts X. anomaly Azores X. anomali Lucskai S. intermedium symbionts X. intermedia X. intermedia Biosys X. bedingii DSMZ 4764 S. glaseri symbionts X. poinarii DSMZ4768 KMD15 S. cubanum symbionts X. cubana + + + - - + - + - - - + + + + ++ + - - ++ - ++ ++ + - + + ++ +/++ - +/+/- - + + + +/+/- + + + ++ +/- - LEGENDS to Table 13. -: not growing at all; +/-: not all J1 develop into fertile adult, +: J1 develops to fertile adult; ++: the new EPB symbiont is retained and was reisolated after several generations. Table 14. Growth of S. carpocapsae and S. feltiae strains on Xenorhabdus symbionts of taxonomically undetermined Steinernema species. Steinernema carpocapsae Mexicana T1 Steinernema feltiae SF 22 Mexicana Symbionts of unknown Steinernema Isolates Z06 Z31/1 3905 S47A H5 FA019 (from R. Gaugler) FA013 HW7 HK2001 HK98 KMD44 - +/- ++ ++ + + + + + - ++ +/++ + ++ +/- ++ + + + + ++ +++ ++ + +++ + + +/+ +++ + ++ ++ - +++ +++ - LEGENDS to table 15. -: not growing at all; +/-: not all J1 develop into fertile adult, +: all J1 develop into fertile adult; ++: the new EPB symbiont is retained and was reisolated after several generations; +++: even more nematodes were produced, than at ++. 62 3.4.2. CONCLUSIONS These data may help to clarify some questions concerning the conception of cospeciation, like what bacterial symbiont might be a better symbiotic partner for a nematode strain, and what might happen in nature when two different symbiotic bacteria mix within the caterpillar. It is known that the symbiotic partners of Steinernema and Heterohabditis inhibit each others growth, and so the nematodes can not develop if mixed in the carcass. The gnotobiological analysis of the Steinernema and Heterorhabditis strain shows that the Steinernema species are more strain specific for their symbionts than the Hetrorhabditis species. The tests prove that the growth of almost all tested Heterorhabditis strains is supported by Photorhabdus strains from different species. The results showed less readiness of the S. carpocapsea and S. feltiae species to be in symbiotic relationship with the others bacterial symbiont, and the symbionts of the Mongolian X. bibionis strains cannot be utilized at all by any S. feltiae strains. It can be concluded that neither S. feltiae nor S. carpocapsae could grow and propagate on the symbiont of other Steinernema species of short dauer phenotype. The gnotobiological analysis might be able to help in the future to understand the molecular bases of the specificity of the symbiotic relations between the EPB and EPN strains. Also it is possible for specific pest controls, better symbiotic relations could be generated as the research moves forward. 63 3.5. FIRST ATTEMPTS TO LABORATORY FERMENTATION OF A NEW STEINERNEMA ISOLATE This chapter is based on the published version for COST 850 (edited by Prof. R.-U. Ehlers). SUMMARY The bacterium symbionts of the Morocco, SP2, Italian and Slovak Steinernema strains of long dauer phenotype were isolated and phenotypically characterized. Each proved to be Xenorhabdus according to cell and colony morphology, dye uptake, catalase negativity, as well as on the basis of their lecitinase, lipase and proteolytic activities. In gnotobiological tests the nematodes from which they had been isolated grew on it and produced fertile progeny in agar plates as well as in liquid culture in our laboratory fermentor. Each bacterium strains produced antibiotics of medium level and proved to be ampicillin resistant. The Morocco strain, however, expressed a low degree of ampicillin resistance. The sequence analysis and taxonomic identification will be accomplished soon. 3.5.1. INTRODUCTION Steinernema species of long dauer phenotypes are a potential tool for controlling scarab grubs. In Hungary the Maybeetle (Melolontha melolontha) is a harmful agricultural pest and entomopathogenic nematode species to control it is needed. We are to produce the effective nematode in liquid culture. As a first step, the bacterial symbionts should be isolated, identified and characterized. Herein we are to publish the first data on the bacterial symbionts of Steinernema sp. Morocco, Steinernema sp. SP2, Steinernema sp. Italy, and Steinernema sp. Slovak strains. 5.5.2. MATERIALS AND METHODS Nematode strains: All strains were kindly provided by the participants of the COST 850 Meeting and Workshop in Debrecen, Hungary. 2002. Namely, the Italian Steinernema strain (“Italy”) was kindly provided by Prof. Trigiani Oreste, (Bari, Italy); the Spanish 64 (SP2) one by Fernando Garcia del Pinto (Barcelona, Spain), the Morocco strain was kindly provided by Ralf-Udo Ehlers (Raisdorf, Germany). 3.5.3. ISOLATION, CHARACTERIZATION OF EPB SYMBIONTS OF SOME NEW STEINERNEMA STRAINS OF LONG DAUER PHENOTYPE Infective dauer juveniles (IJ) freshly harvested from Galleria white traps were collected and washed by centrifugation with sterilized tap water three times. Some were then put in to a drop of physiological saline (M9) solution in a sterile Petri plates and then transferred to 5% chlorox. After 2 minutes the animals were transferred to a series of sterilized M9 drops and finally fragmented by using a sterile platinum wire (Fig 4). Different steps of isolation of entomopathogenic bacterial (EPB) symbionts from infective dauer juveniles (IJ) of entomopathogenic nematodes (EPN). The washing, surface sterilizing, and the removal of the chlorox were made in more steps by direct transfer of the worms from one drop to another. The indicator plates were NBTA, on which the bacterium wanted form black colonies and the agar plate around the colonies was clear. The drop of M9 was diluted and plated on NBTA indicator plate. Blue colonies were picked and retested again. From the positive colony a stock culture was established and frozen. Samples from the stock cultures were then tested for Xenorhabdus primary phenotypes. 3.5.1.1. PHENOTYPIC CHARACTERIZATION OF THE FRESHLY ISOLATED BACTERIAL SYMBIONTS Cell morphologies were examined under light microscopy. Colony morphology and dye uptake: LB, NBTA, LBTA and MacConkey agar plates were used as described by Boemare et al (1996). 3.5.1.2. PHOSPHOLIPASE (LECITINASE TEST ON YOLK AGAR) An egg was surface disinfected with 70% ethanol. Under the laminar flow the yolk was collected aseptically and poured into an equal volume of (0. % NaCl) sterile saline solution. After homogenization, 10% vol/vol of this yolk solution was added to nutrient 65 agar at 45 oC. An opaque halo around the inoculation line meant a positive lecitinase reaction. 3.5.1.3. PROTEOLYSIS ON GELATIN AGAR (FRAZIERS’S METHOD) 12 g/liter of gelatin were added to LB agar powder and the plates were streaked. After the prerequisite incubation, plates were flooded with a solution of 12 g HgCl2; 16 ml of 12N HCl and 80 ml distilled water. A clear halo due to the proteolysis was evidenced. 3.5.1.4. ANTIBIOTICS PRODUCTION 5 µl of an overnight culture of the Xenorhabdus examined were pipetted into the center of an LB agar plate. After a 2-5 days incubation at 30 oC, the plate was overlaid with 5ml soft agar (0.6 g agar/100 ml LB solution) including 1 ml suspension of B. subtilis spores (containing about 108 spores). The plates were incubated overnight at 37 oC. The diameter of the clear (inactivation) zone, as well as that of the bacterium colony were determined 3.5.1.6. AMPICILLIN SENSITIVITY In general, LB plates containing 0, 25, 50, 75, 100, 125, and (150 mg/ml) ampicillin sodium (Sigma) were made. In case of the Morocco strain the ampicillin doses in the LB plates were as follows: 200, 100, 50, 25, 12.5, 6.25, 3.125, 1.563 and 0 ug/ml. An overnight culture (the number of cells per ml was about of about 10-8order of magnitude) was serially diluted to 1:10, 1:102, 1:103, 1:104, 1:105, 1:106, a 0, :107 ratio. 0.1 ml of each dilution was plated into each ampicillin plates. The colonies were then counted and presented as a function of the ampicillin dose. The MIC values were also determined as follows: 5 ml LB liquid cultures containing 200, 100, 50, 25, 12.5, 6.25, 3.125, 1.563 and 0 ug ampicillin sodium /ml respectively were inoculated with 0.1 ml of overnight bacterium culture and incubated overnight at 30 oC. The OD values were determined at 600 nm and presented as a function of the ampicillin dose. The latter was deduced from the previous one. 3.5.1.7. GNOTOBIOLOGICAL TESTS One colony of the stock culture was transferred into 5 ml of LB media. The overnight culture was seeded on one half of a large (of 9 cm diameter) TSY agar plate (in glass 66 Petri plate). Surface sterilized IJ larvae were transferred to the bacterium-free part of the agar plate. The animals were moving to the bacteria. The gnotobiological test was considered positive if the majority of the dauers molted into L4 and grew to fertile adults producing viable progeny. 3.5.2. RESULTS AND DISCUSSION The colonies of the new isolates can be seen and compared on LB, NBTA, egg yolk and tween 20 agar plates, on( Fig 19-22), respectively. Fig. 19: The new Xenorhabdus isolates (Morocco, SP2, Italy, Slovak) and control (X. nematophila (AN6/1), X. poinarii (DSM 4766) strains on Luria Broth (LB) agar plates LEGEND to Fig 19. Phenotypes of the isolates on LB agar plates. Upper row, left to right: Xenorhabdus symbiont of the Spanish SP2, Morocco and Italian strains. Lower row: Slovak, DSM 4466 (reference strain) and X. nematophila AN6/1 reference strain. 67 Fig. 20. Lecitinase activities of my new isolates. Control: DSM 4766 (X. poinarii). LEGEND to Fig.20. Comparison of the lecitinase activities of the new isolates on NBTA agar plates. The new Xenorhabdus isolates: Morocco, SP2, Italy, Slovak and control strain, X. poinarii (DSM 4766) can be detected as cleaning up the agar around the colonies. Fig. 21. Lipase activities of my new Xenorhabdus isolates. LEGEND TO Fig 21. Comparison of the exo-lipase activities of the new isolates on tween 20 agar plates. The new Xenorhabdus isolates: Morocco, SP2, Italy, and Slovak. Control strains: X. nematophila (AN6/1) and X. poinarii (DSM 4766). The lipase decomposes the triglycerides into glycerol and fatty acids, and the Ca-salt of the free fatty acid is precipitated around the bacterium colonies. 68 On the basis of their cell (not shown) and colony morphology (Fig. 19.), catalase negativity, dye uptake (not shown), exo lecitinase (Fig. 20), and exo-lipase (Fig. 21) activities all the four bacterium isolates belong to the Xenorhabdus genus, but definitely different from both the X. nematophilus AN6/1, and X. poinarii reference (control) strains. All of the produce compounds of antimicrobial activity (Fig. 22). Fig. 22. Antibiotic activities of the new Xenorhabdus isolates. Ampicillin resistance: All strains but the Morocco strain proved to be resistant to ampicillin. A low fraction of the cells seems to keep their ampicillin resistance, and so the Morocco strain can grow at 200 µg/ml ampicillin can survive and grow. Gnotobiology: The infective dauer juveniles recover, grow and propagate on TSY agar plates, where they were released on their own symbionts. The nematodes, as expected, can only grow poorly on each other’s bacterial symbionts. 3.5.3. LABORATORY FERMENTATION OF THE MOROCCO STRAIN A new 3.7-liter laboratory fermentor was developed in our laboratory (Fig. 23.). All the Morocco strains can propagate well on their symbiont in the laboratory liquid fermentor. The fermenting conditions were tested with the new Steinernema strain isolated with its own symbiont as well. The results indicate that the Morocco strain has the best fermentation qualities (yield and surival properties of the nematodes) of the strains studied so far. The Morocco strain grown in the fermenter did not prove to be very toxic towards either Manduca sexta orally (J. Marokházi, personal communication) or 69 Melolontha grubs when injected. When injected, the Morocco strain killed Galleria mellonella efficiently (S. Pekár and J. Marokházi, personal communication). Fig. 23: The laboratory fermentation unit. LEGEND to Fig. 24. Laboratory fermentor constructed by Imre Kurucz and Csaba Sisak. 2 X TSY (liquid). medium was used in the fermentor The oxygen was provided by (moderate) stirring and simultaneous aeration. The inoculum for the monoxenic bacteria population was grown on TSY in agar. 3.5.4. CONCLUSIONS The best source of the natural EPB symbionts of an EPN strain is the infective dauer juvenile. Xenorhabdus strains can easily be identified by using the conventional indicator plates. The antibiotics production is an advantage but not a precondition of successful liquid cultures of EPNs. The ampicillin resistance of the Xenorhabdus and Photorhabdus strains is a general feature, but there is significant variation between strains. The unusual behavior of the Morocco strain needs further analysis. The fermentation qualities of a given strain influenced by several factors, but the most important are to have the proper EPB symbionts. The Morocco strain was the easiest to propagate in a 3.7 liter laboratory fermenter. Acknowledgements: This research was supported by a Hungarian National Fundamental research Program (OTKA) T035010 Grant. We would like to express our thanks for the nematode strains to the participants of the COST 850 Meeting and Workshop in Debrecen, Hungary. 2002. For Italian Steinernema strain (“Italy”) to Prof. Trigiani Oreste, (Bari, Italy); for the Spanish (SP2) one to Dr. Fernando Garcia del Pinto 70 (Barcelona, Spain); and Morocco strain to Dr. Ralf-Udo Ehlers (Raisdorf, Germany). We acknowledge the professional help of Prof. A. Szentirmai in the MIC determination, to Dr. Csaba Sisak for his help in operating the fermentor, Mrs. Simon Sára for taking care of the Steinernema strains. The research was also supported by the Research and Extension Centre for Fruit Growing, headed by Dr. Ferenc Inántsy. Thanks to Judit Marokházi and Szilvia Pekár for making the unpublished data available for us. 71 3.6. MOLECULAR, GENETIC AND GNOTOBIOLOGICAL IDENTIFICATION OF NEW STEINERNEMA ISOLATES In this chapter is based on the published version for COST 850 (edited by Prof. R.-U. Ehlers). SUMMARY Thirteen Steinernema strains of long dauer phenotypes were compared on the basis of their PCR – RFLP profiles based on the GeneGel Excel kit analysis. They were also grown on some Xenorhabdus symbionts isolated from S. glaseri, S. arenarium, as well as from unidentified Steinernema isolates, such as SP2, Slovak and “Italy”. They were also intercrossed to study their species differences. We found that one group of strains belong to the S. glaseri group, characterized by very similar RFLP profiles and unlimited cross fertility. They grew on each other is symbionts. Another group comprised the S. arenarium group of rather similar RFLP patterns and gnotobiological similarity, although their cross fertility has shown limitations. Two strains (Morocco, Palestine) definitely belong to two, different species. The taxonomic positions of the SP2 and the Italy strains are still ambiguous. 3.6.1. INTRODUCTION Thirteen Steinernema strains of long dauer phenotypes, potential tools for grub control were studied for their identification within the framework of a COST 850 Workshop. We have studied their growth and development on each others’ bacterial symbionts (gnotobiological analysis), cross fertility and PCR - RFLP profiles obtained by GeneGel excel kit (Blaxter, 1998). PhastSystem PCR RFLP is an excellent method for identifying entomopathogenic nematodes (Filipjev, 1935). On the other hand, the classic species definition is that those individuals which are capable of producing fertile progeny do belong to the same species. We intended to determine which steinernematids of long dauer phenotype belong to the same species and intended to compare their PCR-RFLP patterns by using the GeneGel Excel kit. 72 Considering the species specific symbiosis of Steinernema and Xenorhabdus (bacterium) strains, and as a consequence of it. entomopathogenic nematodes can grow and propagate only on their own symbionts, a precondition for the successful crosses is to find bacteria on which both parental strain could somehow grow and propagate. We have isolated the Xenorhabdus symbionts of several Steinernema strains and tested the others for growth and reproduction. Finally, we found bacterium for each intended crosses. 3.6.2. MATERIALS AND METHODS Bacterium strains: Xenorhabdus bacterium have been isolated straight from the surface sterilized infective dauer juveniles as has been described by Lucskai. Bacteria were tested on indicator plates, for their catalase negativity and for other taxonomic characters. We have isolated Xenorhabdus from the nematode strains KMD15, S. anomali, SP2, Slovak, Morocco and Italy and used also the DSM reference strain of X. poinarii (natural symbionts of S. glaseri). Nematode strains: In our stock collection we have two Steinernema arenarium (anomali) strains: one from N. Simoes (Portugal Azores), one from Ramon Georgis (Biosys, USA). S. glaseri strains NC1 (from Michael G. Klein, USDA-Ohio, USA), NC513, NCLU (from Pierre Abad’s collection via Attila Lucskai) and AZ26 (from N. Simoes, Portugal Azores). The Italian Steinernema strain (“Italy”) was kindly provided by Prof. Trigiani Oreste, (Bari, Italy); the Spanish (SP2) one from Fernando Garcia del Pinto (Barcelona, Spain), the “Polish” strain from Marek Tomalak, Poznan, Poland), the “Slovak” strain from Zdenek Mracek, Ceske Budejovice, Check Republic, The “Russian” strain from Sergei Spiridonov Moscow, Russia, the “Gel” (probably the original Russian isolate) from Z. Mracek; the Morocco strain was kindly provided by Ralf-Udo Ehlers while the “Palestine” one come from Dr. Nair Iraki). For in vitro culturing nematodes, large glass Petri dishes of (9 cms) diameter were filled with TSY agar medium2 and one half was seeded with the proper bacterium. A sterile Sartorius filter of 0.2 µm pore size was put on the bacterium free part of the TSY agar. Surface sterilized IJs were then transferred to filter. Couple of hours later, when the nematodes left the filter and moved toward the bacterial lawn the filter was removed. If the nematodes are in conform to the bacteria, they molt to L4 and grow to fertile adults and propagate. Otherwise they may or may not recover and even if they recover they die 73 or become unfertile. For intercross experiments, small plastic Petri dishes of 3.5 cm diameter were used with TSY agar. A drop of an overnight bacterium culture was dropped in the center of the plates, one virgin (L4) female from the maternal strain, and 1-9 young males from the paternal strain were transferred to the plate by a platinum wire. The Petri plates were closed by using parafilm and put in a “humidity chamber” (e.g. glass Petri plates with water). We observed that the males started to court immediately whoever the females were and usually mated successfully. In the control groups, where the males and the females originated from the same strain, the females became gravid carrying embryos, the majority of which developed to larvae inside their mother (entokia matricida) (Akhurst, 1987, Al-Samarrai et al., 2000). In case of absence of cross fertility the oocytes remained unfertile and no progeny developed. The DNA isolation from single nematodes as well as the PCR amplification of the ITS1 – 5.8S rDNA – ITS2 region of the 18S- 5.8S – 28S rDNA operon was carried out by using the protocol of Triga et al (2000). The molecular analysis was conducted according to the manufacturer’s original protocol, without any modification. Based on our previous studies3, we used four enzymes (AluI, MseI, TaqI and RsaI) to cut the DNA and compare the restriction patterns. 3.6.3. RESULTS AND DISCUSSION Gnotobiology: Morocco strain could grow on almost all Xenorhabdus strains, but the growth was the most efficient on its own symbiont. For crosses only the KMD15 and the Anomali bacterium plates could be used. The data for gnotobiology are summarized in Table 18. Crossbreeding: On the basis of the classic species definition, EPN strains NC1, Kmd15, NC513, NCLU, and AZ 26 do belong to the same (S. glaseri) species, while strains Anomali, Gel, and Russian comprise another species (S. arenarium). On the basis of cross fertility, neither SP2, nor Italy, nor Polish, nor Slovak could be sorted into either of these two species, in spite of some the similarities between the molecular markers (see later). Morocco does definitely belong to another (unknown) species. 74 Table 16. Growth and propagation of Steinernema strains on each others’ symbionts. Xenorhabdus: strains: Steinernema isolates: S. glaseri NC1 NCLU NC513 Kmd15 AZ26 S. anomali Polish Slovak Gel Russian SP2 Italy Morocco X. poinarii Palestine X. sp. X. sp. X. sp. X. sp. X. sp KMD11 Anomali SP2 Italy Morocco +++ +++ +++ +++ +++ - +++ +++ +++ +++ +++ + + + + + + + ++ +++ +++ ++ ++ +++ ++ ++ ++ ++ ++ ++ ++ ++ +++ ++ ++ + + + + + + +++ ++ +++ - - - - Not tested - - LEGENDS to Table 16. Data show that hardly any growth of the majority of the strains could be observed on any artificial symbionts. Of the S. glaseri symbionts only a selected line of Kmd15 bacteria could serve as food for some other strains, while the symbionts of S. anomali (Azores) could moderately be used to grow one or two generations of other steinernematids. Morocco strain could not be grown on any other bacteria, therefore we could not test them in intercross studies. The molecular identification of the bacterial symbionts is in progress. Table 17. The results of cross breeding tests. Maternal strains: NC1 NCLU NC513 AZ26 Kmd15 Ano-mali Gel Russian S. g. NC1 + + + + + - - - NCLU + + + + + - - NC513 + + + + + - AZ26 + + + + + Kmd15 + + + + S. anomali - - - Polish - - Gel - Russian Paternal strains: Sp2 Italy Polish Morocco - - - - - - - - - - - - - - - - - - - - - - + - - - - - - - - - + + + - - - - - - - - - - + - - - - - + + + - - - - - - - - - + + + - - - - SP2 - - - - - - - - + - - - Italy - - - - - - - - - + - - Morocco - - - - - - - - - - - + - LEGENDS to Table 17. The data confirm that EPN strains can be grown and propagate properly on their own natural symbiont or symbionts of other strains of the same species. NCLU, NC513, AZ26 as well as NC I all belong to the same species, S. glaseri, therefore they can utilize each other’s symbiont. In the EPN strains belonging to the S. arenarium species all but the Polish strain can utilize each other’s symbionts, but those of S. glaserii. SP2, Italy, and especially Morocco strain definitely belong to a different species. 75 Molecular analysis: Results of the PCR-RFLP analysis are demonstrated on figures 25, 26 and 27, respectively. Fig 25. GenePhore PCR-RFLP Analysis 1. GenePhore PCR RFLP Analysis M M 7 8 9 10 11 12 M • M = Molecular marker (Azores) / Rsa I • 7 S. anomali (Fragment sizes 120, 130, 190) • 8 Kmd15 / Rsa I (Fragment sizes 130, 210) •9 S. anomali (Azores) / /Mse I ( Fragment sizes 120, 250) • 10 / Kmd15 / Mse I (Fragment sizes 120, 300) • 11 S. anomaly ( Fragment sizes • 12 Kmd15 (Azores) /Taq I 100, 110,125,210, 260) / Taq I (Fragment sizes 100, 110, 125, 150, 230) LEGEND to Fig 25. RsaI, MseI and TaqI PCR-RFLP restriction profiles of the ITS1 – 5.8S – ITS2 regions of the 18S – 5.8.S – 28S operons of entomopathogenic Steinernema strains of long dauer phenotypes belonging to Steinernema arenarium (S. anomali from Nelson Simoes’s collection in Portugal Azores) and Steinernema glaseri (KMD 15). The patterns are individual and reproducible. The RsaI pattern of S. anomali (7) consists of a 120, 130 and 190 Dalton fragments and is different from that of KMD15 (8), which has a profile of 130 and 210 Dalton fragments. Similarly, the two strains also differ in their MseI and TaqI profiles, respectively. Before we started the intercross experiments, based on the RFLP analysis Kmd15 was believed to be belonging to a new species, S. ohioensis (Lucskai and Klein, unpublished) and S. anomali was believed to exist as a separate species. Kmd15, however, can be crossed with all the other four S. glaseri strains (NCI, NCLU, NC 513, AZ26), and - as we could see on Fig. 26. and 27 - their GenPhore PCR-RFLP restriction profiles are also similar. As for our S. anomali strains they could cross freely with each other as well as with the Gel and “Russian” strains. The intercross experiments show that 76 they belong to the same species, (S. arenarium). But the restriction profiles of these strains are not identical. The Polish, Italian and SP2 strains are not identical either. Since the intercross experiments gave also negative results, we suppose that even if all these strains belong to a large S. arenarium group, they are not only geographically, but also sexually isolated. Fig. 26. GenePhore PCR-RFLP Analysis 2. LEGEND TO Fig. 26. Comparison of the TaqI and AluI PCR-RFLP restriction profiles of the ITS1 – 5.8S – ITS2 regions of the 18S – 5.8.S – 28S operons of strains belonging to the S. glaseri (S.g.), S. arenarium (S. a.) groups as well as those of taxonomically unidentified Steinernema strains of long dauer phenotypes from Poland (Polish), Italy, (Italy), Spain (SP2) and Morocco. The AluI profiles of KMD15, NC513, and NCI seem to be identical but AZ26 differs in some smaller fragments significantly. The characteristic “glaseri” pattern, however, cannot be mixed up with any other. The AluI patterns of the Anomali (Biosys) and Gel strains are identical, and that of the Polish strain is very similar to them, almost identical, but the Italian strain, however, seems completely different. The Morocco pattern is completely unique. The TaqI profiles of KMD15, NC513 and AZ26 are unambiguously identical and NCI slightly differ from them. However, all the other RFLP patterns significantly differ from the “glaseri profile”. Those of S. anomali (Biosys) and Gel are similar but not identical and the Polish profile differs from both. The Italy just like the Morocco shows unique pattern. 77 Fig. 27. GenePhore Analysis 3 GenePhore PCR-RFLP Analysis 1. Molecular Marker 2. S. a. (anomali) (Biosys) / MseI 3. S. g. (Kmd15) / MseI 4. S. g. (NC 513) / MseI 5. Steinernema sp. Polish / MseI 6. S. g. NCI /Mse I 7. Steinernema sp. (Morocco)/ MseI 8. S. a. (Gel) / MseI 9. S. g. (Az26) / MseI I / Italy / 10. Steinernema sp. Mse 11. Steinernema sp. (Slovak) / MseI 12. Steinernema sp. (SP2) / MseI 19. Gel / Rsa I 13. S. a. (anomali) (Biosys) / Rsa I 20. S. g. sp.(Az26) / RsaI 14. S. g. Kmd / RsaI / Italy / Rsa I 21 Steinernema sp. 15. S. g. Nc513 / Rsa I I 22. Steinernema .sp (Slovak) / I 16. Steinernema sp. Polish / 23. Steinernema sp.(SP2)/ RsaI 17. S. g. NCI / RsaI Rsa Rsa 18. Steinernema sp. (Morocco) / RsaI 24. Molecular Marker LEGEND to Fig 27. Comparison of the MseI and RsaI PCR-RFLP restriction profiles of the ITS1 – 5.8S – ITS2 regions of the 18S – 5.8.S – 28S operons of strains belonging to the S. glaseri (S.g.), S. arenarium (S. a.) groups as well as those of taxonomically unidentified Steinernema strains of long dauer phenotypes from Poland (Polish), Italy, (Italy), Spain (SP2), Slovak and Morocco. The MseI profiles of KMD15 and NC513 seem identical, and very similar to that of AZ26. This enzyme (as we had found before) 3 could not cut the DNA of S. glaseri NCI. The characteristic “glaseri” pattern, however, cannot be mixed up with any other one. The MseI patterns neither of the anomali (Biosys), nor of Gel are strains different, since the Gel DNA was not very well digested by this enzyme. The Polish strain was almost identical to that of anomali. The Italian strain showed completely different pattern of lots of fragments, but was very similar to that of SP2. The pattern of the Slovak strain was something between the Polish and the Italian, but was definitely different from both of them. The Morocco pattern is completely unique. 78 4. SUMMARY The entomopathogenic nematode (EPN) / bacterium (EPB) symbiotic complexes have great agricultural potential with the key of success in bacterium. The bacterium produces toxins, killing the insect and produce antibiotics to protect the monoxenic EPN / EPB complex against other microorganisms. In my dissertation I aimed to answer some questions related to the taxon specificity of symbiosis and the role of the antibiotics. Several strains available in our stock were tested for antibiotic production. The data demonstrate that different compounds may play a role in the antibacterial action of a EPB and the competition with another EPB strain. Two Xenorhabdus strains, EMA and EMC produce antibiotics extremely effective against Erwinia amylovora (the cause of fire blight disease in apple orchard) both in laboratory and phytotrone tests. These compounds are also effective against Phytophtora and Trichoderma. We are to concentrate and chemically characterize the antibiotics for liquid fermented culture of EMA. The EMC strain produces unusual exo-crystals of carbohydrate nature. The role and exact chemical nature of the crystals are yet to be answered. In different gnotobiological studies it was confirmed that the Steinernema species can grow and propagate only their own symbionts or on symbionts of other nematode species. In this study we have compared large number of strains of Photorhabdus and Xenorhabdus species and we can conclude that the Photorhabdus strains are better source for more Hererorhabditis strains in vivo and in vitro than the Xenorhabdus strains for the Steinernema species. The Steinernema strains are more “picky” in terms of finding proper symbionts. We have isolated the EPB symbiont of the Morocco strain and elaborated a 3.7liter laboratory fermentation technique for growing them. The fermentation technique can be used for other entomopathogenic nematode strains as well. We are recommending a new molecular technique to identify new Steinernema isolates. The GenePhore PCR-RFLP System might be useful for further molecular identification of unknow and known bacterial strains. 79 5. REFERENCES 1. Abadon, M., Grenier, E., Laumond, C., & Abad, P. (1998). A species-specific satellite DNA from the entomopathogenic nematode Heterorhabditis indicus. Genome 1: 148153. 2. Adams, B.J, Burnell, A.M. and T.O. Powers, T. O. (1998). A phylogenetic analysis of Heterorhabditis (Nemata: Rhabditidae) based on Internal transcribed spacer 1 DNA Sequence Data. J. Nematology. 30(1): 22-39. 3. Adams, B.J. & Nguyen, K. B. (2002). Taxonomy and Systematics. In: „Entomopathogenic Nematology” (R. Gaugler ed.)., CABI Publishing, USA, pp. 133. 4. Adams, B.J. (1998). Species concepts and the evolutionary paradigm in modern nematology. J. Nematology 30: 1-21. 5. Akhurst R.J. (1980). Morphological and functional dimorphism in xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes Neoaplectana and Heteror habidilis. J. Gen. Microbiol. 121,303-309. 6. Akhurst R.J. (1980.) Morphological and functional dimorphism in xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes Neoaplectana and Heteror habidilis. J. Gen. Microbiol. 121,303-309. 7. Akhurst, R. J. (1987). Use of starch gel electrophoresis in the taxonomy of the genus Heterorhabditis (Rhabditida: Heterorhabditidae). Nematologica 33: 1 8. Akhurst, R.J. of Boemare, N.E.(1988). Anumerical taxonomic study of genus Xenorhabdus (Enterobacteriaceae) and proposed elevation of the subspecies of X. nematophilus to species Journal of general microbiology , 134: 1835-1845. 9. Akhurst, R.J.(1982). Antibiotic activity of Xenorhabdus spp. Bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae . J.Gen. Microbiol , 128: 3061-3066. 10. Akhurst, R.J., & Boemare, N.E. (1986). A non-luminescent strain of Xenorhabdus luminescens (Enterobacteriaceae). Journal of General Microbiology 132: 1917-1922. 11. Akhurst.,R.J,and G.B.Dunphy.(1993). In : Parasites and pathogens of insects , N.E.Beckage,S.N.Thompson, and B.A.frederici(eds).Academic press, san diego. 12. Al-Samarrai, T.H., Zhang, N., Lamont I. L., Martin, L., Kolbe, J., Wilsher, M., Morris, A J. and Schmied, J. (2000). Simple and inexpensive but highly discriminating method for computer-assisted DNA fingerprinting. J. Clin. Microbiol. 38, 4445-4452 13. Andrássy, I. (1976). Evolution as a Basis for the Systematization of Nematodes. Akadémiai Kiadó, Budapest, 1-287 pp. Idézve: pp. 7-22; 41-44; 45-64; 89-94. 14. Blaxter, M., and D. Bird. (1997). Parasitic nematodes, pp.851-880. In C. elegans II. 15. Blaxter, M.L., P.D. Ley, J.R. Garey, L.X. Liu, P. Scheldeman, A. Vierstraete, J.R. Vanfleteren, L.Y. Mackey, M. Dorris, L.M.Frisse, J.T. Vida, and W.K. Thomas. (1998). Molecular evolutionary framework for the Phylum Nematoda. Nature 392:7175. 16. Boemare, N.E . and Lanois, A. (1996) . Training course for characterization of Xenorhabdus and Photorhabdus phase variation, in entomopathogenic nematodes 80 symbiosis and pathogenicity of the nematode bacterium complexes, Brussels, Luxemburg .pp. 109-114. 17. Boemare, N.E. Akhurst R.J, and Mourant. (1993). \DNA relatedness between Xenorhabdus spp (Enterobacteriaceae) symbiotic bacteria of entomopathogenic nematodes luminecens to a new genes Photorhabdus Gen .nov. Int.J.syst.Bacteriol,43: 340-255. 18. Bovien, P. (1937). Some types of associations between nematodes and insects. Vidensk. Meddeleser fra Dansk Naturahistorisc Forening Khobenhaven 101: 1-114. 19. Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. 20. Burnell, A. M. and Stock, S. P. (2000). Heterorhabditis, Steinernema and their bacterial symbionts – lethal pathogens of insects. Nematology 2: 31-42 21. Buzás, Z., L. Varga. (1995). Rapid method for separation of microsatellite alleles by the PhastSystem. PCR Methods and Applications 4: 380-381. 22. Chitwood, B. G. and Chitwood, M. B. (1937). An Introduction to Nematology. Monumental Printing Company Baltimore, MD, USA, 1-213 pp. 23. Couche, G.A., Lehrbach, Foreg, R.G., Cooney, G.C. Smith, D.R. and Gregson, R.P. (1987.) occurrence of intercellular inclusions and plasmids in Xenorhabdus sp. J. Gen Microbiol. 133,:967,-973. 24. Couche, G.A., Lehrbach, Foreg, R.G., Cooney, G.C. Smith, D.R. and Gregson, R.P. (1987.) occurrence of intercellular inclusions and plasmids in Xenorhabdus sp. J. Gen Microbiol. 133,:967,-973. 25. Curran, J. (1990). Molecular techniques in taxonomy, in EPNs in Biological Control, Gaugler, R., and H. K. Kaya, ed. CRC Press, Boca Raton, FL, chap.3., pp. 63-74. 26. D.L. (1988). The Nematoe Ceanorhabditis elegans. W.B. Wood et al .(eds) cold spring harbor laboratory, New york .The dauer larva .pp.393-414. 27. Dix, I. (1994). Use of second generation Heterorhabditis females for genetic crosses. In: „Genetics of Entomopathogenic Nematode Bacterium Complexes” (Burnell A.M., Ehlers, R.U.,& Masson J.P. eds), COST 812 Biotechnology EUR 15681, Brussels, pp. 190-193. 28. Dix, I., A.M. Burnell, C.T. Griffin, S.A. Joyce, M.J. Nugent, and M.J. Downes. (1992). The identification of biological species in the genus Heterorhabditis (Nematoda: Heterorhabditidae) by cross-breeding second generation amphimictic adults. Parasitology 104: 509-518. 29. Dunphy, G.B, Rutherford, T.A, and Webster, J.M. (1965). Groth and virulence of steinernema glaseri influenced by different subspecies of Xenorhabdus nematophilus, J. Nematol, 17: 476-482. 30. Ehlers, R-U, Stoessel ,S. and Wass, U (1990) . The influence of phase variants of Xenorhabdus spp and Echerichia coli (Enterobacteriaceae ) on the propagation of entomopathogenic nematodes of genera Steinernema and Heterorhabditis .Rev. 31. Ehlers,R.U.and Strauch, O. (1994). Establishment of axinic and Monoxenic cultures (summery of a practical workshop session ). In Biotechnology genetics of entomopathogenic nematode bacterium complexes proceeding of a symposium and workshop held at st. Patrak”s college May nooth, CO. Kidare, Ireland,pp204-207. 32. Ehlers,R-U, Stoessel ,S. and Wass, U (1990) . The influence of phase variants of Xenorhabdus spp and Echerichia coli (Enterobacteriaceae ) on the propagation of entomopathogenic nematodes of genera Steinernema and Heterorhabditis .Rev. 33. Filipjev, I. N. (1934). Miscellanea Nematologia I. Eine Nue Art der Gattung Neoaplectana Steiner nebst Bermekungen über die systematische Stellung der letzenteren, Mag. Parasitol. Institut. Zool. Acad. USSR. 4: 229. 81 34. Filipjev, I. N. (1934). The classification of the free-living nematodes and their rlations to parasitic nematodes. Smitsonian Miscellaneous Collections 89: 1-63. 35. Fodor, A. (1994). Genetic and molecular aspects of dauerlarva formation and recovery. In: „Genetics of Entomopathogenic Nematode Bacterium Complexes” (Burnell A.M., Ehlers, R.U.,& Masson J.P. eds), COST 812 Biotechnology EUR 15681, Brussels, pp. 23-40. 36. Fodor, A., Deák, P.(1986). The isolation and genetic analysis of a C. elegans translocation (szT1) strain bearing an X-chromosome balancer. J. Genet. 64: 143167. 37. Forst S, Dowds B, Boemare N, Stackebrandt E. (1997). Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu. Rev Microbiol.51:47-72. Review 38. Forst S., Nealson, K. H. Boemare, N. and Stackebrandt, E. (1997). Xenorhabdus and Photorhabdus spp.Bugs .Annu. Rev Microbiol 51:47-72. 39. Forst, S. & Clarke, D. (2002). Bacteria-nematode symbiosis. In: R. Gaugler (Ed). Entomopathogenic Nematology. CABI Publishing, New York. pp. 57-77. 40. Forst.S. Nealson, K. H. Microbiol. (1996) Rev. 60:21-43. 41. French Constant,R,Waterfield,N.Daborn,P.Joyce,S,Bennett,H,Au,C.Dowling,A,Bou ndy,S.Reyndds,Sand Clarke,D(2003).Photorhabdus. towards a functionalgenomic analysis of a symbiont and pathogen .FEMS.Microbiol Rev , 26,433-456. 42. Golden, J.W. and Riddle, D.L. (1982). A phenomenone influences larval development in thenematode C.elegans Science 318: 578- 580. 43. Golden, Jew. And DL. Riddle, (1982). A phenomenone influences larval development in thenematode C.elegns Science 318: 578- 580. 44. Gotz, P. Boxman.A.and Borman,H.G.(1981). Interaction between insect immunity and an insect pathogenic nematode with symbiotic bacteria .Proc.R.Soc. Lond ,211 B, 330-350. 45. Griffin, C.T., S.A. Joyce, I. Dix, A. Burnell, and M.J. Downes. (1994). Characterization of the EPN Heterorhabditis (Nematoda: Heterorhabditidae) from Ireland and Britain by molecular and cross-breeding techniques, and the occurrence of the genus in these islands. Fund. Appl. Nematol. 17: 245-253. 46. Han and Ehlers, R. U. (2000). Pathogenicity, development, and reproduction of Heterorhabditis bacteriophora and Steinernema carpocapsae under axenic in vivo conditions. Invertebr Pathol. 75: 55-58. 47. Han. R, Wouts., W.M. Li., I.(1990). Development of Heterorhabditis spp. Strains as characteristics of possible Xenorhabdus luminescens subspecies . Rev. Nematol 13(4): 411-415. 48. Kaya.H.KandGaugler,R(1993).Entomopathogenicnematodes.Ann.Rev.entemol. 38:181-186. 49. Koppenhofer, A.M, Kaya, HK (1999).Ecological charechterezation of Steinernema rarum. J Invertebrate Pathol : 73(1):126-128. 50. Koppenhofer,A.M, Wilson, M. Brown, I , Kaya, H.K, Gaugler, R.(2000). Biological control for white grubs (Coleoptera :Scarabaeidae in anticipation of the establishment of the Japanese beetle in California. J. Econ. Entomol.93: 71 -80. 51. Liu, J. Berry, R.E. and Moldenke, (1997). Phylogenetic relationships of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) inferred from partial 18S rRNA gene sequences. J. Inveretebr. Pathol. 67: 246-252. 52. Luana, S., Stoessel , Schmidt–Peisker, A.J. and Ehlers, R.U (1993). Establishment of monoxenic inocula for scaling up in vitro cultures of the entomopathogenic nematodes Steinernema spp . and Heterorhabditis spp. of Nematologica 39:385-399 . 82 53. Miller, J.H. (1972). Experiment in molecular genetics. Cold spring Harbor Laboratory. N.Y 54. Nealson, K, H, Schmidt, T.M. and Bleakly, B. (1990 Biochemistry and physiology of Xenorhabdus, P 275-284. 55. Poinar, G. O. Jr. (1975). Entomogenous Nematodes. E. J. Brill, Leiden, 1975. 56. Poinar, G. O. Jr. and Georgis, R. (1990). Description and field application of the HP88 strain of Heterorhabditis bacteriophora Rev. Nématol.: . 57. Poinar, G. O. Jr.(1976). Description and biology of a new insect parasitic rhabditoid, Heterorhabditis bacteriophora n. gen. N. sp. (Rhabditida, Heterorhabditidae N. Fam.) Nematologica 21: 463-470. 58. Poinar, G. O. Jr., Jackson, T. and Klein. M. G. (1987). Heterorhabditis megidis sp. N. (Heterohabditidae: Rhabditida) parasitic in the Japonese neetle, Popillia japonica Scarabidaeidae: Coleoptera) in Ohio, Proc. Helminthol. Soc. Wash. 54: 53-59. 59. Poinar, G. O. Jr., Karunakar, G.K., and David, H. (1992). Heterorhabditis indicus, n. sp. Separation of Heterorhabditis spp. by infective juveniles. Fundamental and Applied Nematology 15: 467-472. 60. Poinar, G. O. Jr..and Thomas. G. M. (1978). Laboratory Guide to Insect Pathogens and Parasites. Plenum Press, new York and London. 392 pp. Idézve: pp. 235-280. 61. Poinar, G.O. (1990). Taxonomy and Biology of Steinernmatidae and Heterorhabditidae. In: „Entomopathogenic nematodes in Biological Control”, CRC Press, Boca Raton, Fl, 1990- pp. 23-61. 62. Poinar, G.O. Jr. (1975). Entomogenous Nematodes. E. J. Brill, Leiden, 1975. 63. Riddle, D. L. (1989). The dauer larva. In: The Nematode Caenorhabditis elegans I. W. B. Wood (ed). Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y., USA. pp. 393-412. 64. Riddle, D.L. (1988). The Nematoe Ceanorhabditis elegans. W.B. Wood et al .(eds.) Cold \Spring Harbor Laboratory, New York .The dauer larva .pp.393-414. 65. Steiner, G. (1923). Acleptana kraussei, n. sp., einer in der Blattwespe Lyda sp. Parasitirende Nematodenform, nebst Bemerkungen über das Seitenorgan der parasitischen Nematoden. Zentralbl. Bakteriol. Parasitirendk. Infektionsk. Hyg. Abt. 2: 59, 14-18. 66. Steiner, G. (1929). Neoaplectana glaseri, n.g. n. sp. (Oxyuridae), a new nemic parasite for the Japanese Beetle (Popillia japonica Newm). J. Wash. Acad. Sci., 19: 436-440. 67. Stock, S.P. and Kaya, H.K.(1996). A multivariate analysis of morphometric characters of Heterorhabditis species (Nemata: Heterorhabditidae) and the role of morphometrics in the taxonomy of species of the genus. Parasitology 82: 806-813. 68. Szállás. E., Koch, C. Fodor, A., Burghart, J. Buss, O. Szentirmai, A., Nealson, K.H., and Stackebrandt, E. (1997). Phylogenetic evidence for the taxonomic heterogeneity of Photorhabdus luminescens. Int. J. Syst. Bacteriol. 47: 402-407. 69. Triga, D., H. Pamjav, T. Vellai, A. Fodor, and Z. Buzás. (2000). Gel electrophoretic restriction fragment length polymorphism analysis of DNA derived from individual nematodes, using the PhastSystem. Electrophoresis 20: 1274-1279. 70. Zioni (Cohen-Nissan) S., Glazer, I., and Segal D. (1992). Life cycle and reproductive potential of the nematode Heterorhabditis bacteriophora strain Hp88. J. Nematology 24: 352-358. 83 6. ACKNOWLEDGEMENTS I would like to start out by thanking God, and all the people who helped me in accomplishing my PhD. I am grateful to Professor Gábor Vida, the full member of the Hungarian Academy of Science for all his help and for allowing my work in the Department of Genetics at Eötvös Lóránt University, which is headed by him. I want to express my thanks and appreciation to my supervisor Dr. habil András Fodor, Associate Professor of the Department of Genetics of the Eötvös Lóránt University, who proposed the project to me and helped me in accomplishing it with his guides. My best regards to Professor Sándor Koch, who helped me so much with all his advises and who was like a father to me. To Dr. Károly Márialigeti, head of the Microbiology Department, who also helped in so many ways both professionally and privately. For teaching me how to study and how to prepare for an exam. I would also like to say thanks to Professor Michel Klein for helping with the correction and proofreading of my work. In the microscopic studies and making the pictures, thanks to Dr. Géza Zboray and Attila Kovács Associate Professors at the Zoology Department, who were my Mentors Many thanks for all your help. I have to thank my fellow students in the laboratory for the everyday help they gave me. Andrea Máthé, Sára Simon and Piroska Magyar, also Andrea and Sára as well as Emilia Szállás for previously keeping the nematode and bacterium strains in order, and provided me with the samples whenever I needed. My deepest gratitude to Antonia Völgyi, Katalin Lengyel, Triga Dimitra, Horolma Pamjav, Csaba Ortutay, Takács-Sántha, Tibor Vellai, Bayar Baatar, Antal Vancsó and Kiss Zsuzsanna Halima, who helped me a lot in typing and many things. Last but not least I would like to thank my mother and father and brothers Mohamed Abdorahman and Dr. Yosif and my sisters Fawzia and Turkia and my daughter Mawada for being there for me. Special thanks to my country, Libya, for generously supporting my studies. Thank you all for your time and help! 84