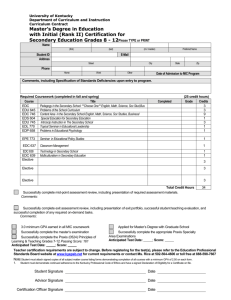
1,2-Dichloroethane
advertisement

1,2-Dichloroethane This Product Safety Summary is intended to provide a general overview of the chemical substance in the context of ICCA Global Product Strategy. The information in the Summary is basic information and is not intended to provide emergency response, medical or treatment information. In-depth safety and health information can be found in the (extended) Safety Data Sheet (e)SDS for the chemical substance. General Statement 1,2-Dichloroethane (commonly known as ethylene dichloride or EDC) is a simple chlorinated organic substance which is found as a colourless liquid at room temperature. It is manufactured by the reaction of chlorine with ethane in the presence of an iron(III) chloride catalyst or the reaction of chlorine with ethylene. It was first prepared in 1794 by the Society of Dutch Chemists. It is used primarily for the production of the vinyl chloride monomer, which is in turn used in the production of polyvinyl chloride (PVC). Over 95% of EDC production is used in PVC manufacture, which is employed in a wide variety of industries. Chemical Identity Name: 1,2-Dichloroethane CAS number: 107-06-2 Molecular formula: C2H4Cl2 Use and Applications EDC is used primarily as an intermediate (over 95% in Europe) in the manufacture of VCM. As EDC and VCM are both hazardous, this production typically takes place in strictly controlled conditions to remove exposure of workers to the substance. EDC is also used in the pharmaceutical industry as a solvent for some specific reactions. Many other uses existed historically, but these have been phased out. All uses are controlled to reduce worker exposure and minimise release to the environment. The substance is not sold or provided for consumer use. Physical/Chemical Properties EDC is a colourless liquid at room temperature. It is often used directly at the production site, though it can also be transported as a liquid. The substance is extremely flammable. Physical state Colour Odour Density Melting temperature Boiling temperature Molecular weight Liquid Colourless Of chlorinated solvents About 1.25 g/cm3 -35 °C 84 °C 98.96 g/mol Human Health Safety Assessment EDC can be toxic to humans, and can cause skin and eye irritation. Inhalation of the vapours can also cause lung irritation. The substance is classified as a probable human carcinogen by IARC. An inhalation study indicates formation of mammary tissue tumours in rats, though the mode of action for this is unknown and epidemiological evidence in humans is lacking. Industrial workers should ensure that they follow the advice found in the extended safety data sheet (eSDS). Environmental Safety Assessment EDC is not classified as harmful to the environment, and environmental exposure is extremely low due to rigorous containment. Exposure Human Health The different uses identified for the substance have been assessed as safe under several regulatory programs. Workers exposure is generally very low due to containment of the substance. Consumers will not come into contact with EDC. The substance has been assessed as safe for industrial use, when the provisions laid down in the eSDS are followed carefully. Environment EDC release into the environment does not normally occur, as it is used within closed systems. Where exposure does occur, this is generally very low. EDC exposure in the environment is stringently regulated and local levels typically monitored. Regulatory Information EDC has been registered under the European REACH Regulation EC/1907/2006 and the substance was found to be safe for the uses identified. The substance was also reviewed under the OECD HPV program (assessment of chemicals produced in high volumes). EDC is a priority substance under the European Water Framework Directive, and thus environmental levels are monitored. Worker exposure to the substance is controlled in many countries by adherence to an OEL. Classification and Labelling The substance is subject to harmonised classification under the EU Classification Labelling and Packaging (CLP) Regulation EC/1272/2008. Industry has adopted a more stringent self-classification, as required under CLP, as follows: Flammable liquid 2 H225 Highly flammable liquid and vapour Carcinogenic 1B H350 May cause cancer Acute toxicity 4 H302 Harmful if swallowed Acute toxicity 3 H331 Eye irritant 2 H319 Causes severe eye irritation Skin irritant 2 H315 Causes skin irritation STOT SE 3 H335 Toxic if inhaled May cause respiratory irritation (Please note that specific labels may differ from the classification above. For complete details on the classification and labelling of 1,2-dichloroethane, consult the SDS.) Conclusion 1,2-Dichloroethane is a hazardous but well controlled substance that is necessary in the production of PVC plastics and is also used as a solvent in the pharmaceuticals industry. The use of this substance has been shown to be safe when exposure is rigorously controlled. Contact Information within Company For further information on this substance or product safety summaries in general, please contact: Xxxxx Xxxxx at xxxxxx@xxxxx.com or visit our website at www.xxxx.com. Euro Chlor (www.eurochlor.org), the European chlor-alkali manufacturers association is a useful repository of information regarding chlorine and can be contacted at eurochlor@cefic.be. Additional information on the ICCA global product strategy can be found here: http://www.iccachem.org/en/Home/ICCA-initiatives/global-product-strategy/ Glossary Acute toxicity Biodegradable Bioaccumulation Carcinogenicity Chronic toxicity Mutagenicity OEL Sensitising Date of Issue xx/xx/xxxx Revised xx/xx/xxxx harmful effects after a single exposure breakdown of materials by a physiological environment accumulation of substances in the environment effects causing cancer harmful effects after long-term repeated exposures effects that change genes Occupational Exposure Limit allergenic