Determination of the heat of hydration of copper(II) sulphate
advertisement

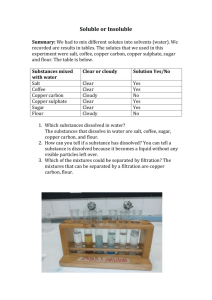
F6/7 Chemistry Practical: Enthalpy of hydration of copper(II) sulphate Objective: To determine the enthalpy of hydration of copper(II) sulphate Group size: Individual Introduction This experiment enables an approximate determination of the enthalpy of hydration of copper(II) sulphate to be made. The enthalpy change when one mole of anhydrous copper(II) sulphate is dissolved in water is first determined. Secondly, the enthalpy of solution of copper(II) sulphate pentahydrate in water is determined. , Thirdly, the enthalpy of hydration of copper(II) sulphate is evaluated by using Hess's Law of Constant Heat (Enthalpy) Summation. ------------------------------------------------------------------------------------------------------------------------Theory: 1. Define the terms 'enthalpy of solution' and 'enthalpy of hydration'. 2. H1 is the enthalpy of solution for anhydrous copper(II) sulphate. H 2 is the enthalpy of solution for copper(II) sulphate pentahydrate. H 3 is the enthalpy of hydration of copper(II) 3. 4. 5. sulphate. Draw an energy cycle to relate (a) the dissolution of anhydrous copper(II) sulphate (b) the dissolution of copper(II) sulphate pentahydrate and (c) the hydration of anhydrous copper(II) sulphate By using the diagram in (2), give an equation to relate H1 , H 2 and H 3 . In procedure A, temperature change, , for the dissolution of anhydrous copper(II) sulphate is measured. Give a formula for the calculation of 'heat given out, Q' by using the temperature change. (Use the following symbols in your formula: mw for mass of water, ma for mass of anhydrous copper(II) sulphate, Cw for specific heat capacity of water, Ca for specific heat capacity of anhydrous copper(II) sulphate, is the temperature change) Express H1 in terms of Q. ---------------------------------------------------------------------------------------------------------------------Procedure A. Determination of heat of solution of anhydrous copper(II) sulphate 1. Using a measuring cylinder, place 100 cm3 of distilled water in a polystyrene beaker and record the initial temperature of the water. 2. By the use of a triple beam balance, weigh 7.5 - 8.5 g of anhydrous copper(II) sulphate, accurate to 1 decimal place, with the aid of a watch glass. 3. Add the anhydrous copper(II) sulphate to the water in the beaker and stir to dissolve it as quickly as possible. 4. Record the highest temperature of the solution. B. Determination of heat of solution of copper(II) sulphate pentahydrate Proceed as in part A, but use 11.0 - 12.5 g of copper(II) sulphate pentahydrate instead of the anhydrous salt. Results for section A and B Anhydrous copper(II) sulphate Mass of watch-glass/g Mass of watch glass + copper(II) sulphate/g Mass of copper(II) sulphate added/g Initial temperature of water/pC Highest/Lowest temperature of solution/oC Change in temperature oC En01_CuSO4_hydrate /p. 1 Anhydrous copper(II) sulphate Calculation 1. Calculate the molar enthalpy of solution of anhydrous copper(II) sulphate, H1 . 2. Calculate the molar enthalpy of solution of copper(II) sulphate pentahydrate, H 2 . 3. Calculate the molar enthalpy of hydration of copper(II) sulphate(VI), H 3 . Discussion 1. There are many assumptions you have made in calculating H1 and H 2 , give at least three of these assumptions. 2. Give at least three errors in this experiment, and suggest methods to minimize the errors. 3. Is this method applicable to evaluate heat of hydration of potassium nitrate? Explain. END En01_CuSO4_hydrate /p. 2