Synthesis and Characterization of Small
advertisement

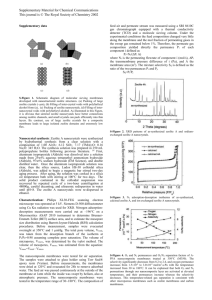
Synthesis and Characterization of Small-Crystal Size Zeolite NaY Wenwen Fu, Jihong Sun* College of Enviromental & Energy Engineering, Beijing University of Technology, Beijing, 100124, P. R. China *Corresponding author, E-mail: jhsun@bjut.edu.cn, Fax: 86-10-67391983 Introduction Studies of composite zeolite are very active subject in petroleum refining processes and have received much attention in recent years [1]. In this report, a novel procedure for the preparation of bi-microporous composite zeoliteβ/Y was being developted in the hydrothermal system by using high silica NaY as precursor in our laboratory, whereas the key work was how to synthesize nanometer Y gel successfully. Meantime, the effects of reaction conditions such as reaction temperature, reaction time and reactant ratio on the composite structure was investigated. Results and Discussion (111) (111) (220) (311) (331) (440) (533) (642) (533) (642) (751) (220)(311) (331) (440) (751) Figure 1 XRD patterns of NaY zeolite crystallized Figure 2 XRD patterns of NaY zeolite crystallized with different time. a-4h, b-8h, c-12h, d-24h with different temperature. a-90℃, b-100℃, c-110℃ NaY zeolite was prepared in hydrothermal conditions in which directing agent was prepared according to the report [2]. Finally, the typical composition of NaY zeolite is following that: Na2O : Al2O3 : SiO2 : H2O = 8:1:10:220. The XRD patterns of NaY zeolite crystallized with different time are given in Figure 1. As can be seen, the results showed that NaY zeolite was prepared with a rather wide range of reaction time from 4 hrs to 36 hrs. When crystallizing with 8 hours, the characteristic peaks (111, 220, 311, 331, 440, 533, 642 and 751) of NaY zeolite was more obvious than that of 4 hours, and their related diffraction intensity was up to the largest. However, with increasing crystallization time (more 12 hrs), the diffraction intensity of thses characteristic peaks was decreased. Figure 2 illustrates the influence of crystallization temperature on the structure of NaY zeolite. The results show that the optimal crystallization temperature of varying between 90℃ to 110℃ was around 100℃ in Figure 2b, which was of the best crystal degree, and corresponding to the uniform particle size with around 150 nm by means of SEM image (not shown). The influence of various reaction parameters are being done. The structure and the synthesis mechanism are further investigated using HRTEM and MAS-NMR. References [1] L. Xu, H. Xu, T. Wu, J. Catalysis, 2006, 27(12): 1149-1158. [2] R. Xu, W. Pang, Chenmistry Zeolite and Porous Materials [M], Science Press, 2004 29 Si