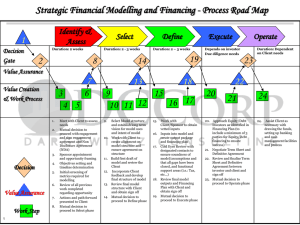
Epoetin_DARF
advertisement

Toll-free 1.866.516.7611 / Fax 877.889.3401 Drug Authorization Request Form Epoetin alfa (Procrit®) Member Information Last Name_____________________________________ First Name ________________________ Sex _________ DOB ______________________ Card # ____________________________ Height __________ Weight _________ Address ________________________________________City _______________ ST ________ Zip_____________ Contact Name _________________________ Home phone (____)____________ Work phone (____)____________ Provider Information Provider Name __________________________________ Phone (____)_______________ Fax (____)___________ Address ________________________________________ City _______________ ST ________ Zip ____________ DEA# _________________________________________Specialty_______________________________________ Medication ___ Procrit (epoetin alfa)___________ Dispense Quantity__________ Refill X ______________month (s) Sig: Treatment of anemia of chronic kidney disease Adults _________ units/kg/week (range: 80-300 units/kg/week) _________ (IV or subcutaneous) in ___________ divided doses per week (range: 2-3) Wt:__________ kg Dose/week: __________units 10,000 units subcutaneous once weekly Other: _____________________________________________________________________________________ Pediatrics (≥3 months or ≥1 month if on dialysis) _________ units/kg/week (range: 50-250 units/kg once to three times weekly) _________ (IV or subcutaneous) in ___________ divided doses per week (range: 1-3) Wt:__________ kg Dose/week: __________units Other: _____________________________________________________________________________________ Treatment of chemotherapy-induced anemia in patients with non-myeloid malignancies Adults 150 units/kg subcutaneous three times weekly Wt:__________ kg Dose: _____________units 40,000 units subcutaneous once weekly Other: _____________________________________________________________________________________ Pediatrics (≥6 months) _________ units/kg/week (range: 25-300 units/kg three to seven times weekly) _________ (IV or subcutaneous) in ___________ divided doses per week (range: 3-7) Wt:__________ kg Dose/week: _________units Other: _____________________________________________________________________________________ Other: __________________________________________________________________________________________ Treatment of anemia associated with zidovudine therapy in patients with HIV Adults 100 units/kg ____________ (IV or subcutaneous) three times weekly for 8 weeks Wt:__________ kg Dose: _____________units 40,000 units subcutaneous once weekly for 8 weeks Other: _____________________________________________________________________________ Pediatrics (≥8 months) _________ units/kg/week (range: 50-400 units/kg two to three times weekly) _________ (IV or subcutaneous) in ___________ divided doses per week (range: 2-3) Wt:__________ kg Dose/week: _________units Other: _____________________________________________________________________________________ For the reduction of allogeneic blood transfusions in surgery patients 300 units/kg/day subcutaneous for 10 days prior to surgery, on day of surgery , and for 4 days after surgery Wt:__________ kg Dose/day: _____________units 600 units/kg subcutaneous once weekly x4 doses on days 21, 14, and 7 days prior to surgery and on day of surgery Wt:__________ kg Dose/week: _____________units Medication ___ Procrit (epoetin alfa)___________ Dispense Quantity__________ Refill X ______________month (s) Sig: Treatment of anemia in patients with non-myeloid malignancies who are NOT receiving chemotherapy 100 units/kg subcutaneous three times weekly Wt:__________ kg Dose: __________units ____________units/kg (range: 150-300 units/kg) subcutaneous three times weekly Wt:_______ kg Dose: __________units Other: __________________________________________________________________________________________ Treatment of anemia in patients with myelodysplastic syndrome 150 units/kg/day subcutaneous Wt:__________ kg Dose/day: __________units 40,000 units subcutaneous ____________ (once or twice) weekly Other: __________________________________________________________________________________________ For the prevention of anemia associated with frequent blood donations 600 units/kg IV twice weekly for 3 weeks starting _____________ days (range: 25-35 days) prior to surgery Wt:__________ kg Dose: __________units Other: __________________________________________________________________________________________ Treatment of ribavirin-induced anemia in patients with hepatitis C 40,000 units subcutaneous once weekly Other: __________________________________________________________________________________________ Treatment of anemia associated with rheumatoid arthritis and rheumatic disease ____________units/kg/week (range: 80-720 units/kg/week) __________ (IV or subcutaneous) in ____________ divided doses per week (range: 2-3) Wt:__________ kg Dose/week: _____________units Other: __________________________________________________________________________________________ Supplies package (no charge): syringes (27g, ½”, 1cc, ndc 08290-3096) qty ____ or 25g, 5/8”, 1cc, ndc 08290-3096-26 qty _____ , sharps disposal unit (regular or large), alcohol wipes (100 per box). PRN refills. DIAGNOSIS: ICD9 CODE: Physician’s Signature______________________________________________________Date_________________________ Drug/Clinical Information 1. Does the patient have anemia associated with chronic kidney disease, non-myeloid cancer, myelodysplastic syndrome, or rheumatoid arthritis/rheumatic disease? Yes 2. Please proceed to Question 3 Please proceed to Question 7 No Coverage not approved Please proceed to Question 5 No Coverage not approved Please proceed to Question 6 No Coverage not approved Have other causes of anemia such as iron deficiency, folic acid and vitamin B 12 deficiencies, occult blood loss, infectious/malignant/inflammatory processes, hematologic disease, aluminum intoxication, hemolysis, and osteitis fibrosa cystica been excluded or appropriately treated? Yes 7. No Is the ferritin concentration ≥100 ng/mL? Yes 6. Please proceed to Question 6 Is the transferrin saturation ≥20%? Yes 5. Please proceed to Question 2 Is the patient receiving epoetin alfa for the reduction of allogeneic blood transfusions or for the prevention of anemia associated with frequent blood donations in patients scheduled for surgery? Yes 4. No Does the patient have anemia associated with zidovudine treatment in patients with HIV or ribavirin treatment in patients with hepatitis C? Yes 3. Please proceed to Question 4 Please proceed to Question 7 No Coverage not approved For patients with chronic kidney disease: a. Has the creatinine clearance been <60 mL/min for at least 3 months? Yes Please proceed to Question b No Coverage not approved No Coverage not approved No Please proceed to Question d b. Is the hemoglobin <11 g/dL? Yes c. Please proceed to Question c Is the patient at least 3 months of age? Yes Please proceed to Question e d. Is the patient at least 1 month of age AND receiving dialysis? Yes e. Please proceed to Question e No Coverage not approved A nephrologist recommended epoetin alfa for this patient. Submit letter of recommendation. Yes Approved for one year pending receipt of current monthly lab values No Coverage not approved For patients with anemia associated with nonmyeloid cancer and who are receiving chemotherapy: f. Is the hemoglobin <10 g/dL? Yes g. Please proceed to Question i No Please proceed to Question g No Coverage not approved Is the hemoglobin >10 g/dL but <12 g/dL? Yes Please proceed to Question h h. Does the patient have symptoms of anemia affecting functional capacity or quality of life such as extreme fatigue or malaise, cold intolerance, tachycardia, congestive heart failure, shortness of breath, severe angina, severe hypotension, or severe pulmonary distress? Yes i. Please proceed to Question k No Please proceed to Question j Please proceed to Question k No Coverage not approved Does the patient have an erythropoietin concentration ≤200 mUnits/mL? Yes l. Coverage not approved Does the patient have documented anemia after chemotherapy treatment within the past year? Yes k. No Has the patient received chemotherapy for at least 2 months? Yes j. Please proceed to Question i Please proceed to Question l No Coverage not approved No Coverage not approved Is the patient at least 6 months of age? Yes Please proceed to Question m m. An oncologist recommended epoetin alfa for this patient. Submit letter of recommendation. Yes Coverage approved for 1 year pending receipt of current monthly lab values No Coverage not approved For patients with anemia associated with zidovudine therapy in patients with HIV: n. Is the hemoglobin <13 g/dL for men or <12 g/dL for women? Yes o. Please proceed to Question o No Coverage not approved Does the patient have an erythropoietin concentration ≤500 mUnits/mL? Yes Please proceed to Question p No Coverage not approved p. Is the patient taking zidovudine doses <4200 mg/week? Yes Please proceed to Question q No Coverage not approved q. Does the patient have symptoms of anemia affecting functional capacity or quality of life such as extreme fatigue or malaise, cold intolerance, tachycardia, congestive heart failure, shortness of breath, severe angina, severe hypotension, or severe pulmonary distress? Yes r. No Coverage not approved No Coverage not approved Is the patient at least 8 months of age? Yes s. Please proceed to Question r Please proceed to Question s An infectious disease specialist recommended epoetin alfa for this patient. Submit letter of recommendation. Yes Coverage approved for 1 year pending receipt of current monthly lab values No Coverage not approved For the preoperative use of epoetin alfa for the reduction of allogeneic blood transfusions: t. Is the hemoglobin 10-13 g/dL? Yes Please proceed to Question u No u. Does the patient have an anemia of chronic disease? Coverage not approved Yes v. Please proceed to Question v No Coverage not approved Is the patient scheduled for major, elective, noncardiac, nonvascular surgery and expected to require >2 units of blood? Yes Please proceed to Question w No Coverage not approved w. Is the patient unable or unwilling to participate in an autologous blood donation program? Yes x. Please proceed to Question x No Coverage not approved A surgeon recommended epoetin alfa for this patient. Submit letter of recommendation. Yes Coverage approved for 1 year pending receipt of current monthly lab values No Coverage not approved For patients with anemia associated with nonmyeloid cancer NOT receiving chemotherapy: y. Is the hemoglobin <11 g/dL? Yes z. Please proceed to Question z No Coverage not approved Does the patient have an erythropoietin concentration ≤200 mUnits/mL? Yes Please proceed to Question aa No Coverage not approved aa. Does the patient have symptoms of anemia affecting functional capacity or quality of life such as extreme fatigue or malaise, cold intolerance, tachycardia, congestive heart failure, shortness of breath, severe angina, severe hypotension, or severe pulmonary distress? Yes Please proceed to Question bb No Coverage not approved bb. An oncologist recommended epoetin alfa for this patient. Submit letter of recommendation. Yes Coverage approved for 1 year pending receipt of current monthly lab values No Coverage not approved For patients with anemia associated with myelodysplastic syndrome: cc. Is the hemoglobin <10 g/dL? Yes Please proceed to Question dd No Coverage not approved dd. Does the patient have an erythropoietin concentration <500 mUnits/mL? Yes Please proceed to Question ee No Coverage not approved ee. Does the patient have symptoms of anemia affecting functional capacity or quality of life such as extreme fatigue or malaise, cold intolerance, tachycardia, congestive heart failure, shortness of breath, severe angina, severe hypotension, or severe pulmonary distress? Yes Please proceed to Question ff No Coverage not approved ff. An oncologist recommended epoetin alfa for this patient. Submit letter of recommendation. Yes Coverage approved for 1 year pending receipt of current monthly lab values No Coverage not approved For the preoperative use of epoetin alfa for the prevention of anemia associated with frequent blood donations: gg. Is the patient scheduled for elective surgery and expected to donate ≥4 units of blood? Yes Please proceed to Question hh No Coverage not approved hh. A surgeon recommended epoetin alfa for this patient. Submit letter of recommendation. Yes Coverage approved for 1 year pending receipt of current monthly lab values No Coverage not approved For patients with anemia associated with ribavirin therapy in patients with hepatitis C: ii. Is the hemoglobin ≤12 g/dL? Yes jj. Please proceed to Question jj No Coverage not approved Does the patient have symptoms of anemia affecting functional capacity or quality of life such as extreme fatigue or malaise, cold intolerance, tachycardia, congestive heart failure, shortness of breath, severe angina, severe hypotension, or severe pulmonary distress? Yes Please proceed to Question kk No Coverage not approved kk. An internist recommended epoetin alfa for this patient. Submit letter of recommendation. Yes Coverage approved for 1 year pending receipt of current monthly lab values No Coverage not approved For patients with anemia associated with rheumatoid arthritis or rheumatic disease: ll. Is the hemoglobin ≤11 g/dL? Yes mm. No Coverage not approved Does the patient have symptoms of anemia affecting functional capacity or quality of life such as extreme fatigue or malaise, cold intolerance, tachycardia, congestive heart failure, shortness of breath, severe angina, severe hypotension, or severe pulmonary distress? Yes nn. Please proceed to Question mm Please proceed to Question nn No Coverage not approved A rheumatologist recommended epoetin alfa for this patient. Submit letter of recommendation. Yes Coverage approved for 1 year pending receipt of current monthly lab values No Coverage not approved Please provide chart notes to document and support statements made above