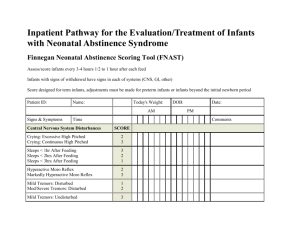
Neonatal Sepsis
advertisement

Neonatal Sepsis A.General comments Bacterial infections most frequently are acquired via the birth canal or nosocomially. The infection almost always is bacteremic (often with seeding of the meanings by way of the blood) and associated with systemic symptoms – a condition referred as neonatal sepsis. Neonatal sepsis, sepsis neonatorum, and neonatal septicemia are terms that have been used to describe the systemic response to infection in the newborn infant. There is little agreement on the proper use of the term, i.e., whether it should be restricted to bacterial infections, positive cultures, or severity of illness. The concept of sepsis as a syndrome caused by metabolic and hеmodynamic consequences of infection is logical and important. In the future, the definition of sepsis in the newborn infant and child will become more precise. At this time, criteria for neonatal sepsis should include documentation of infection in a newborn infant with a serious systemic illness in which noninfectious explanations for the abnormal pathophysiological state are excluded or unlikely. Serious systemic illness in the newborn infant may be caused by perinatal asphyxia, respiratory tract, cardiac, metabolic, neurological or hematological diseases. Sepsis occurs in a small proportion of all neonatal infections. Bacteria and Candida are the usual etiologic agents, but viruses and, rarely, protozoa may also cause sepsis. Blood cultures may be negative, increasing the difficulty in establishing infection etiologically. Finally, infection with or without sepsis may be present concurrently with a noninfectious illness in the newborn infant, child, or adult. B. Incidence. Neonatal sepsis is common in premature infants. About 1% - 4 % of these infants have at least one episode of sepsis during their hospitalization. Sepsis in term infants is rare, occurring in less than 1%. The incidence of neonatal sepsis varies according to definition from 1 –4/1000 live births in developed countries with considerable fluctuation over time and geographic location. Neonatal bacterial sepsis is associated with 10% to 40% mortality and significant morbidity, especially neurological sequel of meningitis. Hospital – to – hospital variability in incidence may be related to rates of prematurity, prenatal care, conduct of labor and environmental conditions in nurseries. Neonatal sepsis may divide into: early onset; late onset; nosocomial. Table 10 classifies the important patterns of neonatal infection by the main features. Attack rates of neonatal sepsis increase significantly in low-birth-weight infants and in the presence of maternal (obstetric) risk factors. Infants <1 month old have immunologic deficient and are predisposed to serious infections. C. Predisposing factors. Premature rupture of membranes (>24 hours), premature labor, maternal fever, UTI, foul lochia, chorioamnionitis, IV catheters (in infant), intrapartum asphyxia, intrauterine monitoring (pressure catheter or scalp electrode). Host risk factors include male sex, developmental or congenital immune defects, galactosemia (Esherichia coli), administration of intramuscular iron, congenital anomalies ( urinary tract, asplenia, myelomeningocele, sinus tracts), omphalitis, and twinning ( especially the second twin of an infected twin). Prematurity is a risk factor for early-onset sepsis. 55 Table 12 Pattern of neonatal infection 56 D.Etiology Bacteria, viruses, fungi, and rarely protozoa may produce neonatal sepsis: 1. Early infection (0 to 4 days of age). Group B streptococci (GBS) and Escherichia coli 60% to 70% of infections. Also Listeria , Klebsiella, Enterococcus, Staphylococcus aureus (uncommon), Streptococcus pneumoniae, group A streptococci. 2. Late infection (>5 days of age). Staph. aureus, GBS, E. coli, Klebsiella, Pseudomonas, Serratia, Staph. epidermidis, Haemophilus influenzae, herpes simplex virus (HSV), enteroviruses. In very low-birthweight infants, Candida and coagulase-negative staphylococci (CONS) are the most common pathogens in late-onset sepsis. Figure 16 Modes of transmission of infections from mother to fetus of infant Maternal Circulation Placenta Fetal Circulation Amniotic Fluid Aspiration Lung Ingestion GI Tract Vaginal Secretion Wound Fetal monitors,vascular access, umbilicus, surgery, necrotizing enterocolitis. D. Pathogenesis. Rarely, inhalation of infected amniotic fluid may produce pneumonia and sepsis in utero, manifested by fetal distress or neonatal asphyxia. Exposure to pathogenesis at delivery and in the nursery or community is the mechanism of infection after birth. The physiologic manifestations of the inflammatory response are mediated by a variety of inflammatory cytokines, principally TNF, interleukin-1 (IL-1), and IL-6, and by-products of activation of the complement and coagulation systems. Studies in the newborn are limited, but it appears that some cytokine production may be diminished, which is consistent with an impaired inflammatory response. However, elevated levels of IL-6, TNF, and platelet-activating factor have been reported in newborn infants with neonatal sepsis and necrotizing enterocolitis. IL-6 appears to be the cytokine most often elevated in neonatal sepsis. 57 F. Signs and symptoms. Presentation may be subtle; thus any febrile neonate (temperature >38° C) must have a septic work-up. Fever may be absent; so watch for symptoms below. 1.The presentation may include irritability, vomiting, poor feeding, poor temperature control, lethargy, apneas spells, and hypoglycemia. 2.May progress to respiratory distress, poor perfusion, abdominal distension, jaundice, bleeding, petechiae, or seizures. 3. Bulging fontanel is a very late sign of neonatal meningitis and Brudzinski’s sign or Kernig’s sign is rarely found. F. Clinical manifestations. Infection is considered in the differential diagnosis of many physical signs in the newborn infant. All of these may have noninfectious explanations. When there is multisystem involvement or when the cardiorespiratory signs are consistent with severe illness, sepsis should be considered. The initial presentation may be limited to only one system, such as apnea, tachypnea with retractions, or tachycardia, but a full clinical and laboratory evaluation will usually reveal other abnormalities. Infants with suspected sepsis should be evaluation for multiorgan system disease. Metabolic acidosis is common. Hypoxemia and carbon dioxide retention may be associated with adult and congenital respiratory distress syndrome (RDS) or pneumonia. The baby who was sucking normally becomes lethargic and unresponsive. He stops sucking at breast. He has a vacant stare and looks ill. Temperature is usually elevated but may be normal or even below normal. The baby loses weight. The liver and spleen may become enlarged. Episodes of apnea may be the only manifestations of sepsis in the preterm babies. Diarrhea, vomiting, abdominal distension and jaundice are other cardinal manifestations. Marked pallor, ashen gray color, cold extremities with absent peripheral pulsations and fall in blood pressure indicate a state of shock, precipitated by the infection. Many newborn infants with infections don’t have serious systemic physiologic abnormalities. Many infants with pneumonia and infants with stage II NEC do not have sepsis. In contrast, stage III NEC is usually accompanied by the systemic manifestations of sepsis, and urinary tract infections (UTIs) secondary to obstructive uropathy may have hematological and hepatic abnormalities consistent with sepsis. Each infant should be reevaluated over time to determine physiologic changes secondary to infection have reached a moderate to severe level of severity that is consistent with sepsis. Late manifestations of sepsis include signs of cerebral edema and/or thromboses, respiratory failure as a result of acquired respiratory distress syndrome (ARDS). Pulmonary hypertension, cardiac failure, renal failure, liver disease with hyperbilirubinemia and elevated enzymes, prolonged prothrombin time (PT) and partial thromboplastin time (PTT), septic shock, adrenal hemorrhage with adrenal insufficiency, bone marrow failure ( thrombocytopenia, neutropenia, anemia), and disseminated intravascular coagulation (DIC). G. Laboratory findings The laboratory evaluation for neonatal sepsis should include: 1. Complete blood count (neutropenia or an elevated ratio of immature to total neutrophils thrombocytopenia are suggest sepsis) and repeat in 5 hours, ESR and C-reactive protein 58 2. A lumbar puncture (LP) (LP for cell count, protein, glucose, and culture. Consider sending CSF for viral studies). 3. Cultures of blood, urine, and any other site as indicated. Latex agglutination test for pneumococcus, E. coli, H. influenzae, group B streptococci, and meningococcus in blood, urine, and CSF is done even though the usefulness is questionable. Negative latex agglutination tests do not rule out infection, but positive results may help guide therapy. 4. Chest radiograph 5. Gastric aspirate (at the time of delivery) for neutrophil count, Gram stain, and culture. 6. Gram stain and culture of a tracheal aspirate, if the infant is intubated. Associated laboratory findings. Hypocalcaemia, hypoglycemia, hyponatremia, prolonged PT and PTT and DIC. Documentation of infection is the first diagnostic criterion that must be met. It is important to note that infants with bacterial sepsis may have negative blood cultures so that other approaches to identification of infection should be taken. Test to demonstrate an inflammatory response include erythrocyte sedimentation rate, C-reactive protein, haptoglobin, fibrinogen, nitroblue tetrazolium dye, and leukocyte alkaline phosphatase. In general, these tests have limited sensitivity and are not helpful. Only the total WBC count with differential and the ratio of immature to total neutrophils provide immediately predictive information compared with age standards. Neutropenia is more common than neutrophili in severe neonatal sepsis, but it also occurs in association with maternal hypertension, neonatal sensitization, periventricular hemorrhage, seizures, surgery, and possibly hemolysis. An immature neutrophil-total neutrophil ratio 0,16 or greater suggests bacterial infection. Criteria for the magnitude of physiologic change in newborn infants with sepsis are not currently defined but should be consistent with the systemic effects of endogenous mediators on one or more organ systems. For example, the effect of sepsis from pneumonia on respiratory function should exceed the local damage in the lung. Thus, a work up for sepsis should include the laboratory studies: 1. Evidence for infection. Culture from a normally sterile site (blood, CSF, other).Demonstration of a microorganism in tissue or fluid. Antigen detection (urine, CSF). Maternal or neonatal serology (syphilis, toxoplasmosis). Autopsy. 2. Evidence for inflammation. Leukocytosis, increased immature/ total neutrophil count ratio. Acute-phase reactants: CRP, ESR. . Cytokines, IL-6. Pleocytosis in CSF, sunovial, or pleural fluid. Disseminated intravascular coagulation: fibrin split products. 3. Evidence for Multiorgan System Disease. Metabolic acidosis: pH, pCO2. Pulmonary function: , pO2 , pCO2. Renal function: BUN, creatinine. Hepatic injury/function: bilirubin, SGPT,SGOT, ammonia, PT, PTT. Bone marrow function: neutropenia, anemia, thrombocytopenia. , pCO2 Treatment. 59 Treatment of neonatal sepsis may be divided into antimicrobal therapy for the suspected or known pathogen and supportive care. 1. Should be tailored to age of onset, clinical setting, and initial findings. 2. There should be NO DELAY in antibiotic therapy. Begin empiric therapy after cultures are obtained or before cultures if any delay is anticipated. There are isolates of Streptococcus pneumoniae that are resistant to penicillin and cephalosporins. 3. As of 1997, the American Academy of Pediatrics recommends adding vancomycin with or without rifampincin to these regimens when meningitis or pneumococcal sepsis is suspected until sensitivities are known. Never use rifampincin alone since resistance can rapidly develop. 4. Empiric early (0 to 4 days old). Ampicillin 50 mg/kg/day (100 mg/kg/day in meningitis) divided 12 hours IV and gentamicin 5 mg/kg/day divided 12 hours IV. Or cefotaxime 50mg/kg q 12 h + ampicillin as above (preferred by some authors). Ceftriaxone is an alternative to cefotaxime. 5. Empiric late (5 days old). Depends on cause (for example methicillinresistant Staph. aureus outbreak requires vancomycin). General guidelines include ampicillin 100 to 200 mg/kg/day divided Q6h plus cefotaxime 150 mg/kg/day IV Q8h), or ampicillin-gentamicin as above usually adequate. Ceftriaxone 100 mg/kg/day IV Q12h is an alternative to cefotaxime. Dose and interval for administration of antibiotics commonly used in newborns vary with the birth weight and postnatal age (see Table 13). 6. Repeat cultures in 24 to 48 hours. In meningitis, repeat LP every day until clear. 7. Other. Fluids, electrolytes, and glucose should be monitored carefully with correction of hypovolemia, hyponatremia, and hypoglycemia and limitation of fluids if there is inappropriate antidiuretic hormone secretion. Shock, hypoxia, and metabolic acidosis should be identified and managed with inotropic agents, fluid resuscitation, and mechanical ventilation. Adequate oxygenation of tissues should be maintained because support of ventilation is frequently necessary for respiratory failure caused by congenital pneumonia, persistent fetal circulation, or adult RDS (shock lung). Refractory hypoxia and shock may require extracorporeal membrane oxygenation, which has reduced mortality rates in full-term infants with septic shock and persistent fetal circulation. Hyperbilirubinemia should be considered for infants who cannot sustain enteral feedings. 60 Table 13 Doses of antibiotics 61 for the newborn DIC may complicate neonatal septicemia. Platelet counts, hemoglobin, PT, PTT, and fibrin split products should be monitored. DIC may be treated by management of the primary sepsis, but if bleeding occurs, DIC may be treated with fresh frozen plasma, platelet transfusions, or whole blood. Because neutrophil storage pool depletion has been associated with a poor prognosis, a number of clinical trials of polymorphonuclear replacement therapy have been conducted, with variable. Results: sepsis that is unresponsive to antibiotics with persistent neutropenia may be an indication for granulocyte transfusion. The use of granulocyte-macrophage colony-stimulating factor (GM-CSF) is under investigation. Treatment with intravenous immunoglobulins (IVIG) containing specific antibiotics is currently under clinical investigation. Currently, granulocyte colony-stimulating factor (GCSF), and IVIG are experimental therapies of undetermined value. It is important to remember that nonbacterial infectious agents can produce the syndrome of neonatal sepsis. Herpes simplex infection requires specific treatment, as no systemic Candida infection. Such agents should be considered in all patients who have negative cultures but whose condition continues to deteriorate despite supportive care and the use of broad-spectrum antibiotics. 62 INTRAUTERINE INFECTIONS According to literature more than 10% children were infected intrauterine. Most infection agents are viruses in antenatal period. In intranatal period both viruses and bacteria can cause infections. Clinical manifestation takes place in 5-50% children. Severity and clinical signs of intrauterine infections depend on the phase of embrio- or fetogeneses more than on the property of infection agent. If the infection develops at the early stage of pregnancy, severe defects of fetus (embriopathy) are formed. That leads to miscarriage. If infection occurs after 8-12 weeks of gestation embrio/fetopathy may be compatible with survival. But there is some changes which lead to miscarriage or severe disease or lethal outcome. If infection develops after II or III trimester of pregnancy, the signs of generalized infection or pathology of organs (hepatitis, myocarditis, meningoencephalitis, meningitis, horioretinitis) may be present. The most typical symptoms of intrauterine infections which can manifest in early neonatal period are summarised in table 14. Table14 NONSPECIFIC SIGNS OF INTRAUTERINE INFECTIONS IN NEWBORN General Cardiovascular System Fever, hypothermia "Not doing well" Poor feeding Scleroema Pallor, mottling, cold, clammy skin Tachycardia Hypotension Gastrointestinal System Abdominal distention Vomiting Diarrhea Hepatomegaly Respiratory System Apnea, dyspnea Tachypnea, retraction Flaring, grunting, cry Bradycardia Central Nervous System Irritability, lethargy Tremors, seizures Hyporeflexia, hypotonia Abnormal Moro reflex Irregular respirations Full fontanel High-pitched cry Cyanosis Hematologic System Jaundice Splenomegaly Pallor Renal System Oliguria Petechiae, purpura Bleeding Taking into consideration the totality of all clinical signs in intrauterine infection of various etiology the term "TORCH-syndrome” is used. „T” stands for toxoplasmosis „O” stands for „other” includes-siphylis, listeriosis, viral hepatitis, variocelar and other „R” stands for „rubella”; „C” stands for cytomegalovirus; „H” stands for herpes. 63 Lately a tendency to increased mixed infection has been observed. In some cases (more frequently at the intrauterine infection) classic TORCH-syndrome is absent at first days of a child’s life, but symptoms of infection develop during observations of a newborn. The early diagnosis promotes separation of the baby to the higher risk group. Definition of higher risk group of intrauterine infection Besides children, who actually have intrauterine infection, the newborn is considered to belong to the risk group, if the mothers have: -pathology of obstetric-gynecological history (miscarriages, stillborn, newborns with defects, children, that died in early age.) -pathology of the pregnancy and delivery (miscarriage, placenta previa, oligohydramnion, membranes rupture.) -diseases of the urogenital system (erosion of cervix uterus, endocervicitis, colpitis, salpingoophoritis, urine infection) -infection diseases during pregnancy accompanied by rash, jaundice, hepatosplenomegaly, lymphadenopathy, hyperthermia, ARVI -immunodeficiency (AIDS) -blood transfusions -state after transplantation, immunosuppressive therapy Intrauterine infection may be suspected if the newborn has the following clinical, laboratory or instrumental signs: -growth retardation (intrauterine hypotrophy) -congenital defects (including congenital heart disease) or stigmata -nonimmune hydrops of fetus -micro- or hydrocephaly -skin exanthemas -fever on the first day of life -neurological damages and seizures during some days after birth -interstitial pneumonia, myocarditis or carditis -keratoconjuctivitis -cataracts and glaucoma - „inflammatory” changes in the blood count (thrombocytopenia, anemia, increase of erythrocyte sedimentation rate, leucopenia, lymphocytosis, monocytosis and erythroblastosis) during the first days of life -changes of neurosonography (cysts, diffuse or periventricular brain calcification). Revealing of two or more sings permits to relate a newborn to the higher risk group of intrauterine infection. The intrautering infections are most probable in preterm infants. Diagnosis Laboratory investigation for TORCH should be made in newborn from a high risk group. The methods of diagnosis are divided into two groups: direct (that reveal microorganisms or viruses in biological fluids or tissue) and indirect (specific immune response of the fetus or newborn) methods. Direct methods : -microscopy - discovering of viral or bacterial antigens 64 - polymerase chain reaction (PCR) - cultural method. The direct methods of diagnosis discover agents in biological fluids or biopsy, but their sensitivity depends on the type of microorganism and quality of equipment and reagent. Recently PCR is a hypersensitive method of diagnosis of all infection, but the „gold standard” of diagnosis of herpes and rubella is cultural method, that of syphilis is Wassermann reaction. Microscopy is less sensitive than other methods. Indirect methods include serological (immune enzymes analysis) IEA: discovering specific IgG and IgM antibodies. This method in newborn is worse than in older children due to particularities of immune response and presence of maternal antibodies in the newborn. But the method is simple. We should take into consideration the following data: -investigation should be made before hemotranfusion; -results of newborn should be compared with maternal results; -presence of specific IgG in concentration equal to or less than maternal concentration confirms transplacental passage of maternal antibodies without infection. Principles of diagnosis and management at birth. The newborns with typical manifestation of TORCH-syndrome and babies from the higher risk group should be observed with intensive care. If dysadaptation occurs, the syndromal or supportive therapy should be performed. If a child is from the mother with primary syphilis or genital herpes, they are referred to special clinic. Others may go to family doctor observation. If a child has infection such as herpes type I or II, variocellar, enterovirus, virus hepatitis A or B, the child must be sent to hospital immediately due to epidemiological danger. Characteristic of most widespread intrauterine infections Cytomegalovirus infection 1. Etiology cytomegalovirus (CMV) 2. Predisposing factors and pathogenesis The fetus is infected from mother with primary CMV infection in 50% cases. Only 1 % of fetuses are infected when pregnant women have a recurrence of a previous CMV infection. The severe neurological complications in children occur if a mother has only primary CMV infection. If CMV infection is acquired from delivery, breast feeding, blood transfusions, it does not lead to severe neurological damages. Probability of development of primary CMV infection: every second women has an infected fetus. If a newborn has congenital CMV infection, his probability of severe neurological complications is one forth. 3. Clinical manifestations. In most cases CMV infection are manifested without clinical symptoms. The late complications such as deafness, minimal brain dysfunction and other minimal brain dysfunctions may develop in 10-15% cases. Syndrome of congenital CMV infection is discovered very rare. Typical sings are: small for date baby, hemorrhagic rash, thrombocytopenia, anemia, jaundice, hepatosplenomegaly, microcephalus, chorioretinitis. Small body weight with hepatosplenomegaly and persistent jaundice is more frequent. Intra-and postnatal infections lead to latent disease, as a rule, with a decreasing immune system. Incubate 65 period lasts 3 weeks. After that hepatomegaly, lymfadenopathy and pneumonia may occur. There may be an atypical lymphocyte in blood analysis. The severe interstitial pneumonia or CMV infection due to blood transfusions may lead to lethal outcome in preterm infants. 4. Diagnosis. CMV in higher titer may secrete with urine or saliva in children who have congenital CMV infection. Virusological investigation should be made. Immunoenzyme analysis helps to discover specific IgM to CMV infection in umbilical serum and peripheral blood. Particles of virus may be discovered during electromicroscopy of the saliva, urine precipitation or biopsy of the liver. We can find typical gigantic cells with insertions (owl’s eye) during cytological investigation of urine precipitation or liver tissue. DNA-discovering. Presence of increased level of specific anti-CMV antibodies IgG at the age from 6 till 12 weeks of life confirms congenital CMV infection 5. Treatment and prevention. The efficient specific treatment is absent. Anti-CMV immunoglobuline „Cytotek” (Biotest pharma, Germany) may be used for decrease of viremia. It is prescribed intravenously 2ml/kg every 2 days or 4ml/kg every 4 days until the decrease of clinical symptoms of CMV infection. CMV is relatively insensitive to acyclovir. Gancyclovir is currently undergoing assessment in infants with congenital CMV infection; the intravenous dosage is 6 mg/kg every 12 hrs for 6 wk. Early results indicate that Gancyclovir treatment reduces the excretion of virus and to determine severity of CMV infection the supplementary diagnostic investigation is necessary: neurosonography, skull X-ray, computer tomography, chest and bone X-ray, biochemical analysis ??moderate the postpartum disease??. However, viral excretion often resumes once treatment is stopped. Yet, there is no information whether one course of Gancyclovir will alter the long-term progression of congenital CMV disease. A frequent side effect of Gancyclovir therapy is bone marrow suppression and therefore it should only be used for newborns as part of an approved investigational protocol. Dynamic observation of hearing is necessary due to development of deafness. Infants with CMV infection may be infected from contacting people. It is not advisable to let pregnant women care for ill children. Use of CMV-negative blood for transfusion. Prevention of development of congenital CMV infection is impossible. Nowadays there are investigation for creation of vital anti-CMV infection vaccine. Herpes 1. Etiology The two types of simplex herpes virus VSH-1 and VSH-2 play a crucial role in pathogenesis of neonatal herpes infection. VSH-2 leads to severe generalized disease and lethality in newborn 2. Predisposing factors and pathogenesis Transplacental passage that leads to miscarriage or congenital defects is rare. The child becomes infected during passage in birth canal due to contact with secretion of vagina. 3. Epidemiology. The likelihood of transmission of herpes from a pregnant woman to her fetus or newborn depends on the factor whether the woman has a primary HSV genital infection during the pregnancy or whether she has a recurrence. If she has a primary infection, the risk of neonatal HSV infection is 44%; for a recurrence, the 66 risk is only 3%. HSV-2 is the agent responsible for more than 75% of all neonatal infections, although genital lesions are present at parturition in less than 10% of cases, and a history of gestational genital HSV infection is obtained from only a minority of mothers who deliver infected infants. There is rising way of this infection if the membrane rupture takes place. Very dangerous risk of the primary genital herpes is present during delivery in mother. The hospital spread may be present too. 4. Clinical sings depend on the form of the disease. Course without symptoms is rare. There are local or generalized herpes- infections. Local: herpes affects eyes or skin. Generalized form is similar to sepsis. Isolate pathology of CNS (herpes meningoencephalitis), may be accompanied by fever, lethargy, poor appetite, hypoglycemia, DIC-syndrome or irritation of CNS, seizures. Typical vesicular elements on the skin or in the mouth are very important signs for herpes-infection. CNS abnormalities are similarly frequent and include microcephaly often associated with atrophy of the brain or cystic encephalomalacia, involvement of the spinal cord, chorioretinitis and microphthalmia. In addition, hepatitis and calcifications in the lungs and adrenal glands have been reported. 5. Diagnosis Microscopically gigantic polynuclear cells -infection of mother (vagina, cervix) -investigation of antibodies. 6. Prevention -caesarian section (if the mother has genital herpes on the III trimester of pregnancy) before membrane rupture or during 4 hours after membrane rupture. 7. Management. -isolation of the newborn -taking into consideration severe complications of herpes, sick infants and infants with risk of development of herpes are subject to management of acyclovir -dose of medicine and course of treatment depend on clinical form of decease and laboratory and instrumental investigations -isolated skin damage requires acyclovir of 30 mg/kg daily divided into 3 infusions during 10-14 days -Acyclovir is prescribed in dose 60-90mg/kg if a generalized form, pathology of CNS or ophthalmoherpes take place at least 14 days. The ointment with acyclovir is used simultaneously. The main complication of intravenous acyclovir therapy is renal dysfunction as a result of crystal formation in the tubules; therefore, serum creatinine levels should be obtained every 3 days during the treatment. The dosage should be reduced if the creatinine level begins to rise above twice of the initial value. The administration of two renal toxic drugs at the same time, e.g., acyclovir and gentamicin, should be performed with care. -interferon increases effects of acyclovir treatment -some clinics have a positive effect from using immunoglobuline with higher titer of antiherpes antibodies (1/320000 and more). -expedient introduction of immunoglobulin during first 5 days of disease with acyclovir intravenously or intramuscular We can use: -reaferon (-2-interferon) in suppositories 100-150 thousands U/kg twice during 5 days per rectum 67 -interferon (-2-interferon plus vitamin C and E) in suppositories 250wu/kg twice a day during 5 days per rectum 7. Prognosis. Despite the result of effective acyclovir therapy, disseminated neonatal HSV infections and localized encephalitis have considerable morbidity and mortality rates. The outcome is improved by early identification and prompt initiation of treatment before the onset of disseminated disease, shock, or coma. The prognosis for neonatal encephalitis caused by HSV-1 appears to be better than that for HSV-2 infection. Varicella-zoster virus Varicella-zoster virus (VZV) is one of the seven human herpesviruses. Primary infection leads to chickenpox. When pregnant women contract chickenpox they may also infect the fetus during the viremic phase. The precise risk to the fetus has been difficult to establish, but it appears to be about 25%. However, not every infected fetus has congenital varicella syndrome. Clinical manifestation of congenital Varicella - Zoster virus fetopathy (stigmata) is the following: o Damage to sensory nerves ( skin lesions, hypopigmentation) o Damage to Optic nerve and Lens (microphthalmia, cataracts, choriorctinitis, optic atrophy) o Damage to Brain : encephalitis, microcephaly, hydrocephaly, calcification, aplasia of brain , hypoplasia of an extremity motor/sensory deficits, deep tendon reflexes are absent, anisocoria, Horner syndrome, anal or vesical sphincter dysfunction o Damage to Cervical or Lumbosacral Cord Diagnosis. Viral DNA can be detected in tissue samples by a hybridization technique. Some infants have VZV specific IgM antibody detectable in the cord blood sample, although the IgM titer drops quickly postpartum. The diagnosis can be made antenatal by obtaining a fetal blood sample for VZV-specific IgM titer. However, VZV has not been cultured from the amniotic fluid. Treatment and prevention. The damage caused by fetal VZV infection does not progress postpartum, an indication that there is no persistent viral replication. Thus, antivira treatment of infants with congenital VZV syndrome is no indicated. Since the varicella vaccine is now available for general use, VZV fetopathy will be preventable by immunization of VZV-susceptible young women. Vaccination of the same group of women is also indicated because morbidity and mortality rates of gestational chickenpox are considerable. Smallpox 1. Etiology - virus of smallpox. 2 .Epidemiology. The 90% women have immunity to smallpox. Congenital or postnatal smallpox is rare. 3. Clinical manifestation. If the mother has smallpox in the I or II trimester of pregnancy, the scar skin, defects of extremities, eyes, brain and loss of body weight may be present in the newborn. 68 4. Diagnosis. Congenital smallpox is characterized by specific IgM-antibodies or IgG. Postnatal Smallpox -Typical eruption with false polymorphism. -Virus in vesicular contents. -Fluorescent antibody technique. -Microscopy - gigantic cells. 5. Tactics. -Isolation for 7 days from the rash onset. -Specific treatment is absent. -All children need polyvalent immunoglobulin (sick children and contact ones.) -Newborns with clinical manifestation of smallpox are required acyclovir 45mg/kg during 10 days. Rubella. 1. Etiology. RNA-virus 2. Epidemiology. Transplacental way. Virus has higher teratogenic effects, than others. About 85% women in fertile age have immunity to rubella due to disease in childhood. Infection in fetus occurs if a woman has rubella during pregnancy. Risk to become infected depends on the term of gestation. If the fetus is infected during 8 weeks of gestation the risk is 80%. The fetus has multitude congenital defects. Maternal antibodies protect a child during first 6 month of the child's age. Firm immunity is formed (20-25 years) 3. Clinical signs. Classic triad: -congenital heart disease (OAD, stenosis of pulmonary arterials, septal defects) or myocarditis; eyes pathology (glaucoma, chozioretinitis, bi- or unilateral cataracts) associated with microphthalmia; defect of hearing (sensorineural deafness) During the further development, pathology of the liver and CNS, immunodeficiency, anemia, pneumonia, thrombocytopenia may be. 4. Diagnosis -newborns with congenital rubella may secrete virus during some years in urine, mucous -specific IgM or IgG antibodies 5. Management -specific treatment is absent -isolation of newborn -treatment of all anomalies -all medical personnel has to have antibodies to rubella due to vaccine When acute rubella infection is documented in a pregnant woman during the first half of gestation, there is a high likelihood of fetal infection with multiple fetal stigmata. Therefore, prenatal diagnosis is recommended so that termination of pregnancy can be considered. 69 Viral hepatitis 1. Etiology. -hepatitis A, B, C, D virus 2. Epidemiology. Hepatitis A virus -transmission to newborn is possible if the mother has acute period or blood transfusion. -if anti-HAV-IgM is present in the newborn stool, hospitalization of the baby is necessary. -if the mother has had acute hepatitis A during III trimester, the human immunoglobulin 0.02 ml/kg intramuscular introduced to newborn. Hepatitis B virus Transplacental transmission is rare. Infection of newborn may be during or after birth. For protection of HBV-infections the specific immunoglobulin (HbIg) and Heptavax are used . The risk of infection in newborn is higher if the mother has Hepatitis B at III trimester of pregnancy 3. Clinical manifestation. -Most of newborns get HbsAg from mother with asymptomatic disease. -Some (1-3%) of newborns have jaundice, rise of transaminase. -HbsAg-carriers have a risk of hepatocellular carcinoma. 4. Diagnosis. -HbsAg is a marker of acute HBV-infection. -HbeAg with HbsAg is a marker of massive infection. -Anti-HBs is a marker of replication of virus and immune competence for HBV. -Anti-HBc is a marker of chronic HBV-infection. 5. Tactics -maternal screening for HbsAg -immunoglobulin Hbig 1.5 ml during first hours of life is necessary for newborn from HbsAg-positive mothers -hepatitis B vaccine 0.5 ml during 7 days of life and 6 months -isolation of newborns with hepatitis B -no need toisolate a mother-carrier and a newborn -observation of newborn having got Hbig or vaccine during 15 months of life -HbIg and vaccine protect 90% newborn. HIV 1. Etiology. Human Immunodeficiency Virus 2. Epidemiology. Transplacental or intrapartum transmission or acquired way. Risk of fetal HIV infection from mother is 13-39%: -transmission may be intrauterine or via breast feeding -caesarian section does not reduce risk of HIV-infection -risk of fetus-infection may be decreased if the mother gets azidothimidin during pregnancy - may be transmited through hemotransfusions. 3. Clinical manifestation 70 -very frequently the newborns do not have clinical signs of disease during first days after birth -clinical signs of AIDS are: repeated infections, severe diarrhea, decreased growth development 4. Diagnosis. - Specific IgA after 3 mo of life; - PCR 5. Tactics. -adequate parenteral feeding -prophylactic of pneumocystic infection -immunoglobulin intravenously 400 mg every 4 weeks -antiviral drugs (DDI and others) may be used only for a newborn with clinical manifestation of HIV-infection. -the women receive oral Zidovudine therapy (100 mg five times daily) throughout the remainder of gestation. During labor the drug is administered intravenously; a loading dose of 2 mg/kg is given over 1 hr followed by continuous infusion of 1 mg/kg/hr until delivery. The newborns receive 6 wk of antiviral therapy (Zidovudine syrup at 2 mg/kg every 6 hr), beginning 8-12 hr postpartum. This result in a 67.5% relative risk reduction. -antibiotics for accompanying infections. Toxoplasmosis 1. Etiology Toxoplasma gondii Only primary (asymptomatic) infection of the mother leads to congenital toxoplasmosis. The risk of fetus pathology is 15% at I trimester and 65% at the end of pregnancy. 2. Clinical manifestation Classic triad: -hydrocephaly, chorioretinitis and brain calcifications may be present in the newborn infected during I trimester of pregnancy. -some newborns have clinical manifestation of sepsis, jaundice, hepatosplenomegaly and deafness -a newborn that was infected at the end of pregnancy does not have clinical signs of disease. 3. Diagnosis. -Seibin-test. -IFA -PCR -cultural method 4. Management. Infants should be treated for 1 yr. For the first 6 mo, oral pyrimethamine (1-2 mg/kg/24hr for 2 days, then 1mg/kg/24hr for 2 mo, then 1 mg/kg/24hr Monday, Wednesday and Friday, sulfadiazine or triple sulfonamides (100 mg/kg loading dose, then 100mg/kg/24hr in two divided doses), and calcium leukovorin (5-10 mg/24hr Monday, Wednesday and Friday) should be administered. 71 During the second 6 mo this regimen is continued or given in ?alternatem? months with spiramycin (50mg/kg twice a day). Syphilis 1. Etiology. Spirochete: Treponema pallidum. Spectrum of clinical signs varies: from asymptomatic to multyorganic damage. 2. Clinical manifestation Rhinitis, rashes, hepatosplenomegaly, jaundice, lymphadenopathy, nephritis, pseudopalsy, stillbirth, premature, developmental delay, fetus hydrops may be present. 3. Diagnosis. -serological test or screening test (Wasserman's reaction) - immunofluorescence reaction - reaction immobilization of treponema pallidum - microscopy “ dark field” (mucous from the nose, liquor) - morphological investigation of placenta -X-ray -lumbar puncture -blood count 4. Treatment. -Penicillin 100 SU/kg 6 times daily during 28 days. -Prolonged penicillin derivates (extencillinum, retargen) 100 SU/kg 1 time a week –cours3 injections. -Novocain-Penicillin- 100SU/kg 2 times a day 28 days Chlamidiosis 1. Etiology. Intracellular parasites: Chlamidia trachomatis. 2. Clinic -conjunctivitis with onset from 2-d week of life, may have chronic form -pneumonic syndrome (interstitial pneumonia, bronchiolitis ) at the age of 3-16 weeks. 3. Diagnosis. -chest X-ray (emphysema) -blood count (eosinophillia) -pO2 level increases, but pCO2 is normal -IgM (serum) -microscopy -cultural method 4. Treatment. -Conjunctivitis: 0,5% erythromycin eye unguent. -Pneumonic syndrome (azitromycin, macropen & other). -Inductors of interferon The general data of viral, parasitic and spirochetal agents associated with fetal and infany morbidity and mortality with late sequels are showed in table 15. 72 Table 15 Viral, Parasitic, and Spirochetal Agents Associated with Fetal and Infant Morbidity and Mortality Agents Fetus Neonatal Disease Rubella virus Aborti on Low birth weight, hepatosplenomegaly, petechiae, osteitis Cytomegalovi rus Anemia, thrombocytopenia, hepatosplenomegaly jaundice, encephalitis Varicellazoster virus Low birth weight, chorioretinitis, congenital chicken pox or disseminated neonatal varicella zoster Mild febrile disease, exanthemas, aseptic meningitis, disseminated disease, multiple organ involvement (CNS. liver, heart), gastroenteritis Congenital poliomyelitis Disseminated disease, multiple organ involvement (lung, liver, CNS) vesicular skin lesions, retinopathy Asymptomatic HBAg positive infection, low birth weight, rarely Picorna viruses Aborti Coxsackie on virus Echovirus Poliovirus Herpes simplex virus Hepatitis B virus Aborti on Aborti on 73 Congenital Defects Heart defects, microcephaly cataracts, microphthalmi a Late sequelse Possible congenital heart disease, myocarditis Neurological deficits Deafness, mental retardation, thyroid disorders, diabetes, degenerative brain tissue autism Microcephaly, Deafness, microphthalmi psychomotor a retinopathy retardation, cerebral calcification Limb Fatal outcome hypoplasia, due to secondary cortical infection atrophy, skin lesions Paralysis Possible microcephaly, retinopathy, intracranial calcifications Neurological deficits Chronic hepatitis, persistent HB acute hepatitis Ag positive AIDS Human immunodefici ency virus Parvovirus BI9 AIDS Borrelia Bergdorf Toxoplasma gondii Rash, prematurity, cortical blindness Low birth weigh, hepatosplenomegaly, jaundice, anemia Stillbir Skin lesions, rhinitis, th hepatosplenomegaly, Hydro jaundice, osteitis, ps anemia fetalis Aborti Hepatosplenomegaly, on jaundice, anemia, poor feeding, vomiting Aborti Low birth weight, on jaundice, anemia, petechiae, heart failure, hepatosplenomegaly mega esophagus, encephalitis Treponema pallidum Malaria Trypanosoma cuzi (Chagas disease) Stillbir th Hydrop s fetalis Stillbir th Aborti on Anemia 74 Hydrocephalu s. microcephaly Chorioretinitis, mental retardation Interstitial keratitis, frontal bossing, saber shins, tooth changes Cataracts Myocarditis, achalasia Neonatal hyperbilirubinemia Neonatal hyperbilirubinemia is a condition that is characterized by excessive concentration of bilirubin in the blood. There are two types of hyperbilirubinemia: unconjugated, which can be physiologic or pathologic in origin, and conjugated, which always stems from pathologic causes. Both types may lead to jaundice, neurotoxic concentrations of unconjugated bilirubin can cause kernicterus. Source of bilirubin. Bilirubin is derived from the breakdown of heme-containing proteins in the reticuloendothelial system. The major heme-containing protein is red blood cell hemoglobin. Hemoglobin released from senescent RBCs in the reticuloendothelial system is the source of 75% of all bilirubin production. One gram of hemoglobin produces 34 mg of bilirubin. The other 25% of bilirubin is called early labeled bilirubin. It is derived from hemoglobin released by ineffective erythropoiesis in the bone marrow, from other heme-containing proteins in tissues and from free heme. Normal bilirubin metabolism Bilirubin is a bile pigment formed from the degradation of heme that is mainly derived from red blood cell destruction (75%), but also from ineffective red blood cell production (25%). The intermediary product of hemoglobin degradation - biliverdin – is converted to bilirubin through a reduction reaction (hemoglobin → verdoglobin → biliverdin → bilirubin). Fat-soluble bilirubin normally circulates in plasma bound to albumin, from which it is transported into hepatocytes. Conjugation with glucuronide converts bilirubin to a water -soluble product, which is excreted into the bile. Conjugated bilirubin in the biliary tree enters the gastrointestinal tract and is then eliminated from the body in the stool. Physiologic hyperbilirubinemia. The serum unconjugated bilirubin level of most newborn infants rises over 34.2 μmol/l in the first week of life. This level usually rises in full-term infants to a peak of 103 to 137 μmol/l by 3 days of age and then falls. A rise to 205.2 μmol/l is in the physiologic rfnge. In premature infants, the peak may be till 205.2 μmol/l on the fifth day of life, possibly rising over 265.5 μmol/l without any specific abnormality of bilirubin metabolism. Levels under 34.2 μmol/l may not be seen until 1 month of age in both full-term and premature infants. This normal jaundice is attributed to the following mechanisms: A. Increased bilirubin production due to 1. Increased RBC volume/kilogram and decreased RBC survival (90-day vs.120 –day) in infants compared with adults. 2. Increased ineffective erythropoiesis and increases turnover of nonhemoglobin heme proteins. B. Increased enterohepatic circulation caused by high levels of intestinal betaglucuronide, preponderance of bilirubin monoglucuronide rathe than diglucuronide, decreased gut motility with poor evacuation of bilirubin- laden meconium. C. Defective uptake of bilirubin from plasma caused by decreased ligandin and binding of ligandin by other anions. D. Defective conjugation due to decreased UDPG-T activity. E. Decreased hepatic excretion of bilirubin. 75 Nonphysiologic hyperbilirubinemia may not be easy to distinguish from physiologic jaundice. Diagnosis of the ethyology of hyperbilirubinemia see at figure 17. Breast-milk jaundice is of late onset. By day 4, instead of the usual fall in the serum bilirubin level, the bilirubin level continues to rise and may reach 342-513 μmol/l by 14 day of age.If breast-milk feeding is stopped, the bilirubin level will fall rapidly in 48 hours. The mechanism of true breast-milk jaundice is unknown. Breast-feeding jaundice. Infants who are breast-fed have bilirubin levels slightly higher in the first 3 to 4 days of life than bottle-fed infants. The main factor thought to be responsible for breast-feeding jaundice is a decreased intake of milk that leads to increased enterohepatic circulation. 76 CLINICAL JAUNDICE MEASURE BILIRUBIN Bilirubin ≥205.2 μmol/l and infant < 24 hours old Bilirubin < 205.2 μmol/l and infant < 24 hours old follow bilirubin COOMBS’ TEST Positive COOMBS’ Negative COOMBS’ Identify antibody •RH •ABO •Kell,etc. DIRECT BILIRUBIN Direct Bilirubin > 34.2 μmol/l concider: Direct Bilirubin < 34.2 μmol/l •hepatitis •intrauterine, viral or toxoplasmatic infection •biliary obstruction •sepsis HEMATOCRIT •galactosemia •alpha-1-antitrypsin deficiency • cystic fibrosis •tyrosinosis •cholestasis normal or low high •hyperalimentation (polycythemia) • syphilis • hemochromatosis RBC MORPHOLOGY RETICULOCYTE COUNT ABNORMAL •spherocytosis •elliptocytosis •stomatocytosis •pyknocytosis • ABO incompatibility •Red cell enzyme deficiency • alpha thalassemia • drugs •DIC NORMAL • enclosed hemorrhage • increased enterohepatic circulation • breast milk • hypothyroidism • Crigler-Najjar syndrome • infant of diabetic mother • RDS • asphyxia • infection • Gilbert’s syndrome • galactosemia Figure 17 Diagnosis of the ethyology of hyperbilirubinemia 77 Causes of neonatal hyperbilirubinemia are following: Overproduction Fetomaternal blood group incompatibility ( e.g. RH, ABO) Hereditary spherocytosis, eliptocytosis, somatocytosis Nonspherocytic hemolitic anemias G-6-PD deficiency and drugs Pyruvate-kinase deficiency Other red-cell enzyme deficiencies Alpha talassemia Acquired hemolysis due to vitamin K3, nitrofurantoin, sulfonamides, antimalarias, penicillin, oxytocin, infection Petechiae Hematomas Pulmonary, cerebral, or occult hemorrhage Underproduction Galactosemia Familial nonhemolytic jaundice types 1 and 2 ( Crigler – Najjar sundrom) Gilberts’ disease Hypothyroidism Tyrosinosis Hypermethioninemia Drugs and hormones – Novobiocin, Pregnanediol, Lucey-Driscoll syndrome Prematurity Hypopituitarism Anencephaly Biliary atresia Dubin-Johnson and Rotor’s syndrome Choledochal cyst Cystic fibrosis (inspissated bile) Tumor or band ( extrinsic obstruction) Mixed Sepsis Intrauterine infections 9 Toxoplasmosis, Rubella, CID, CMV, Herpes simplex, Syphilis, hepatitis Respiratory distress syndrome Asphyxia Infant of diabetic mother Severe erythroblastosis fetalis Uncertain mechanism Chinese, Japanese, American Indian infants Breast-milk jaundice 78 Erythroblastosis fetalis results from the transplacental passage of maternal antibody active against red blood cell antigens of the infant, leading to an increased rate of red cell destruction. It continues to be an important cause of anemia and jaundice in newborn infants despite the development of a method of prevention of maternal isoimmunization by Rh antigens. Although more than 60 different red blood cell antigens capable of eliciting an antibody response in a suitable recipient have been identified significant disease is associated primarily with the D antigen of the Rh group and with incompatibility of ABO factors. Rarely, hemolytic disease may be caused by C or E antigens or by other red blood cell antigens, such as Cw, Cx, Du, K (Kell), M, Duffy, S, P, MNS, Xg, Lutheran, Diego, and Kidd. Anti-Lewis antibodies do not cause disease. The most commonly involved antigen is Rho (D) - from the blood group system. Rh incompability is associated with extravascular hemolysis. ABO blood group antigens are less commonly involved. In utero, if the anemia is severe (usually involving Rh incompability) the fetus (infant) will exhibit the signs and symptoms of hydrops fetalis. Nonphysiologic jaundice should always be suspected when the umbilical cord serum bilirubin concentration is elevated, when the clinical appearance of jaundice is within the first 24 hours of life, or when the conjugated fraction of the serum bilirubin concentration exceeds 2 mg/dl (34μmol/L). Hemolytic Disease of the Newborn Due to Rh Incompatibility. The Rh antigenic determinants are genetically transmitted for each parent and determine the Rh type and direct the production of a number of blood group factors (C, c, D, d. E, e). Each factor can elicit a specific antibody response under suitable conditions; 90% are due to D antigen, the remainder to C or E. Pathogenesis. Isoimmune hemolytic disease from D antigen is approximately three times more frequent in whites than in blacks. When Rh positive blood is infused into an Rh negative woman through error or when small quantities (usually more than 1 ml) of Rh positive fetal blood containing D antigen inherited from an Rh positive father enter the maternal circulation during pregnancy, with spontaneous or induced abortion, or at delivery, antibody formation against D may be induced in the Rh negative recipient mother. Once immunization has occurred considerably smaller doses of antigen can stimulate an increase in antibody titer. Initially, a rise of antibody in the 19S gamma globulin fraction occurs, which later is replaced by 7S (IgG) antibody; the latter readily crosses the placenta, causing hemolytic manifestations. Hemolytic disease rarely occurs during a first pregnancy, since transfusions of Rh positive fetal blood into an Rh negative mother tend to occur near the time of delivery, too late for the mother to become sensitized and transmit antibody to the infant before delivery. The fact that 55% of Rh positive fathers are heterozygous (D/d) and may have Rh negative offspring and that only 50% of pregnancies have fetal-to-maternal transfusions reduces the chance of sensitization, as small family size, in which the opportunities for its occurrence are fewer. Finally, the capacity of Rh negative women to form antibodies is variable; some producing low titers even -after adequate antigenic challenge. Thus, the overall incidence of isoimmunization of Rh negative mothers as risk is low, with antibody to D detected in less than 10% of those studies, 79 even after five or more pregnancies; only about 5% ever have babies with hemolytic disease. When mother and fetus are also incompatible with respect to group A or B, the mother is partially protected against sensitization by the rapid removal of Rh positive cells from her circulation by her anti-A or anti-B, which are IgM antibodies and do not cross the placenta. Once the mother has been sensitized, the infant is likely to have hemolytic disease. There is a tendency for the severity of Rh illness to worsen with successive pregnancies. The possibility that first affected infant after sensitization may represent the end of the mother's childbearing potential for Rh positive infants argues urgently for the prevention of sensitization when this is possible. Such prevention consists of injection into the mother of anti-D gamma globulin immediately following the delivery of each Rh positive infant. Clinical Manifestations. A wide spectrum of hemolytic disease occurs in affected infants born to sensitized mothers, depending on the nature of the individual immune response. The severity of the disease may range from only laboratory evidence of mild hemolysis (15% of cases) to severe anemia with compensatory hyperplasia of erythropoietic tissue, leading to massive enlargement of the liver and spleen. When the compensator capacity of the hematopoietic system is exceeded profound anemia results in pallor, signs of cardiac decompensation (cardiomegaly, respiratory distress), massive anasarca and circulatory collapse. This clinical picture, termed hydrops fetalis, frequently results in death in uterus or shortly after birth; it may also occur from other non immune causes. The severity of hydrops is related to the level of anemia and the degree of reduction in serum albumin (oncotic pressure), which is due in part to hepatic dysfunction. Alternatively, heart failure may increase right heart pressures, with the development of general edema. Failure to initiate spontaneous effective ventilation because of pulmonary edema or bilateral pleural effusions results in birth asphyxia; following successful resuscitation, severe respiratory distress may develop. Petechiae, purpura and thrombocytopenia may also be present in severe cases, reflecting decreased platelet production or the presence of concurrent disseminated intravascular coagulation. Jaundice is usually absent at birth because placental clearance of lipid soluble unconjugated bilirubin but in severe cases bilirubin pigments stain the amniotic fluid, cord, and vernix caseosa yellow. Icterus is generally evident on the 1st day of life because the infant's bilirubin-conjugating and excretory systems are unable to cope with the load resulting from massive hemolysis. Indirect-reacting bilirubin therefore accumulates postnatal and may rapidly reach extremely high levels, which represent a significant risk of bilirubin encephalopathy. There may be a greater risk of developing kernicterus from hemolytic disease from comparable non hemolytic hyperbilirubinemia, although the risk in an individual patient may be a function only of the severity of illness (anoxia, acidosis and so on). Hypoglycemia occurs frequently in infants with severe isoimmune hemolytic disease and may be related to hyperinsulinism and hypertrophy of the pancreatic islet cells in these infants. Infants born after intrauterine transfusion for prenatally diagnosed erythroblastosis may severely affected since the indications for the transfusion are evidence of already severe disease in uterus (e.g. hydrops, fetal anemia). Such infants usually have very high (but extremely variable) cord levels of bilirubin, which reflects the severity hemolysis and its effect on hepatic function. Infants treated with intra umbilical vein 80 transfusions in uterus may have a benign postnatal course, if anemia and hydrops resolve prior to birth. Anemia from continuing hemolysis may be masked by the prior intrauterine transfusion, and the clinical manifestations of erythroblastosis may be superimposed upon various degrees of immaturity resulting from spontaneous or induced premature delivery. Rh hemolytic disease may manifest as hemolytic anemia, icterus gravis, hydrops fetalis. Congenital Hemolytic Anemia. This is the mildest but also rarest form of RH hemolytic disease jaundice is generally absent. Onset is the end of the first week or later. Icterus Gravis. When hemolysis in uteri is less intense, deep jaundice appears during the first 12 to 24 hours. Progressive anemia and hepatosplenomegaly are invariably present. Some may have purpura. Incidence of kernicterus is high. Those who to survive are often left with sequel. Hydrops Fetalis. This is the severest form of the disease. The infant is often preterm and may die in uteri or shortly after birth from severe anemia and CCF. He is markedly edematous with effusion in serous cavities and has gross hepatosplenomegaly. In case of stillbirth, the fetus may be macerated. Placenta is always large and edematous. Etiology. The causes of hydrops fetalis are varied and include: a. Severe chronic anemia due to: - Isoimmunisation (i.e. Rh incompatibility); - Homozygous L-thalassemia; - Twin-to-twin of fetomaternal transfusion. b. Cardiac disease, such as: - Structural defects; - In uterus closure of the foramen ovale; - Paroxysmal atrial tachycardia. c. Hypoproteinemia. d. Intrauterine infection, including syphilis, toxoplasmosis, cytomegalovirus. e. Chromosomal disorders (e.g. Turner syndrome) f. Idiopathic causes. Pathophysiology. The exact pathophysiology is unknown, but the central factor in the development of hydrops fetalis appears to be severe chronic anemia with loss of oxygen-carrying capacity, leading to hypoxia and acidosis. A contributing factor is hypoproteinemia, which together with anemia causes the development of congestive heart failure, edema, pleural effusions. All of these problems contribute to the respiratory distress seen at birth. Signs and symptoms include: congestive heart failure, pallor, ascites, pleural effusions; peripheral edema, hepatosplenomegaly. Laboratory findings include: anemia, hypoproteinemia, hypoxia, acidosis. Diagnosis is based on the maternal medical and obstetric history (e.g. Rh sensitization of the mother) and the clinical and laboratory findings. Therapy is aimed at correction the anemia and treating the congestive heart failure and respiratory distress. In addition appropriate treatment should be provided for associated etiologies and conditions. 81 Kernicterus is a severe neurological condition associated with very high levels of unconjugated bilirubin in the blood. Kernicterus is characterized by yellow staining of the basal ganglia and hippocampus, which is accompanied by widespread cerebral dysfunction. Causes. Kernicterus occurs when free bilirubin crosses the blood-brain barrier and enters the brain cells. Normally, unconjugated bilirubin is bound tightly to albumin, which prevents bilirubin from crossing the blood-brain barrier. Free bilirubin exists when the amount of unconjugated bilirubin exceeds the binding capacity of albumin. Bilirubin also may enter the brain at low concentrations owing to placement from the albumin-binding site by another compound (e.g. sulfa drug), which leads to an increased free bilirubin concentration, or because of disruption of the blood-brain barrier by sepsis, asphyxia, acidosis, or infusion of hyperosmolar solutions. It usually occurs when serum indirect bilirubin of more than 20 mg persists in blood for more than 24 hours. If the level of unconjugated bilirubin increases more than 428-496 mkmol/L, kernicterus occurs 30% of the full-term infants; more than 518-684 mkmol/L 70% of the mature infants. But if the newborns are premature, kernicterus occurs when serum indirect bilirubin of more than 171-205 mkmol/L persists in blood. Kernicterus causes a complex of neurological symptoms, including lethargy or irritability, hypotonia, opisthotonus, seizures, mental retardation, and hearing loss. Clinical manifestations start on 2nd to 6th day. Child is toxic, jaundiced, stops sucking and starts getting opisthotonic fits, and later generalized convulsions. The damage usually becomes gradually obvious (if not fatal) and by the age of 3 years child has bilateral choreoathetosive movements, mental retardation, dystonic spasms,-vertical gaze paralysis and deafness. Other neurological signs proportionate to the widespread involvement may be present. Table 16 Criteria of Hemolytic Disease in neonates by L.S. Persianinov. Symptoms Degree of severity of he disease disease I II III Anemia (Hb) 150 g/L 150-100 g/L < 100 g/L Jaundice 85,5 μmol/L 85,5-36,8 (serum μmol/L bilirubin) Edema/dropsy No edema or Edema little edema >136,9 μmol/L Hydrops fetal Laboratory Data. Prior to treatment, the direct Coombs test is usually positive. Anemia is usual. The cord blood hemoglobin varies, usually proportionally to the severity of the disease; with hydrops fetalis it may be as low as 30-40 g/L. Alternatively despite hemolysis it may be within the normal range owing to compensatory bone marrow and extramedullary hematopoesis. The blood smear usually shows polychromasia and a marked increase in nucleated red blood cells. The reticulocyte count is increased. The white blood cell count is usually normal but may be elevated, and there may be thrombocytopenia in severe cases. The cord bilirubin is usually between 3 and 5 mg/dL (51-86 µmol/L); only rarely is there a substantial 82 elevation of direct-reacting (conjugated) bilirubin. The indirect-reacting of bilirubin rises rapidly to high levels in the first 6 hr of life. After intrauterine transfusions the cord blood may show a normal hemoglobin concentration, negative direct Coombs test, predominantly type 0Rh negative adult red cells, and a relatively normal smear. Marked elevation of both indirect- and direct-reacting bilirubin levels has been reported in these infants. Diagnosis. The definitive diagnosis of erythroblastosis fetalis requires demonstration of blood group incompatibility and of corresponding antibody bound to the infants red blood cells. Antenatal Diagnosis. In Rh negative women a history of previous transfusion, abortion, or pregnancy should suggest the possibility of sensitization. Expectant parent's blood types should be tested for potential incompatibility, and the maternal titer of IgG antibodies to D should be assayed at 12-16, 28-32, and 36 wk. The presence of measurable antibody titer at the beginning of pregnancy, a rapid rise in titer, or a titer of 1:64 or greater suggests significant hemolytic disease, although the exact titer correlates poorly with the severity of disease. If a mother is found to have antibody against D at a titer of 1:16 or greater at any time during a subsequent pregnancy, the severity of fetal disease should be monitored by amniocentesis, percutaneous umbilical blood sampling (PUBS), and ultrasonography. If there is a history of a previously affected infant or a stillbirth, an Rh positive infant is usually equally or more severely affected than the previous infant, and the severity of disease in the fetus should be followed. Assessment of the fetus may require information obtained from ultrasound, amniocentesis, and PUBS. Real-time ultrasound is used to detect the progression of hemolysis from mild to severe, with hydrops defined as skin or scalp edema, pleural or pericardial effusions, and ascites. Early ultrasonography signs of hydrops include organomegaly (liver, spleen, heart), double bowel wall sign (bowel edema), and placental thickening. There may then be progression to polyhydramnios, ascites, pleural or pericardial effusions, and skin or scalp edema. If pleural effusions precede ascites and hydrops by a significant period of time, causes other than fetal anemia should be suspected. Hydrops is invariable when fetal hemoglobin is less than 5 g/dL, frequent when under 7 g/dL and variable between 7 and 9 g/dL. Real time ultrasound predicts fetal well-being by the biophysical profile whereas Doppler ultrasound assesses fetal distress by demonstrating increased vascular resistance. If there is ultrasound evidence of hemolysis (hepatosplenomegaly), early or late hydrops or fetal distress, an amniocentesis or PUBS should be performed. Amniocentesis is used to assess fetal hemolysis. Amniocentesis is performed if there is evidence of maternal sensitization (titer 1:16), if the father is Rh positive, or if there are ultrasound signs of hemolysis, hydrops or distress. That may be performed as early as 18-20 wk of gestation. Postnatal Diagnosis. Immediately after the birth of any infant to an Rh negative woman, blood from the umbilical cord or from the infant should be examined for ABO blood group, Rh type, packed cell volume and hemoglobin, and reaction of the direct Coombs test. If the 83 Coombs test is positive, baseline serum bilirubin should be measured, and a commercially available red blood cell panel should be used to identify red blood cell antibodies that are present in the mother's serum, both tests being done not only to establish the diagnosis but also (to ensure the selection of the most compatible blood for exchange transfusion should be necessary. The direct Coombs test is usually strongly positive in clinically affected infants and may remain so for a few days up to several months. Prevention of Rh Sensitization. The risk of initial sensitization of Rh negative mothers has been reduced from between 10 and 20% to less than 1% by intramuscular injection of 300 mg of human anti-D globulin (1 ml of Rh0GAM) within 72 hr of delivery or abortion. This quantity is sufficient to eliminate approximately 10 ml of potentially antigenic fetal cells from the maternal circulation. Large fetal-to-maternal transfers of blood may require proportionately more Rh 0 GAM. Rh 0GAM, administered at 28-32 wk and again in birth (40 wk), may be more effective than a single dose. The use of this technique, combined with improved methods of defecting maternal sensitization and quantization the extent of the fetal-to-maternal transfusion, plus the use of fewer obstetric procedures that increase risk of such fetal-to-maternal bleeding (versions, manual separation of the placenta, and so on), should further reduce the incidence of erythroblastosis fetalis. Table 17 Suggested Maximum Indirect Serum Bilirubin Concentrations in Preterm Infants** (Berman R.E., Kliegman R.M., 1996; Shabalov N.P., 1997) Birth weight Uncomplecated Complicated* (g) mg/dL µkmol/L Mg/dL µkmol/L <100012-13 222 10-12 171 1250 125014-16 257 12-14 222 1499 150016-20 291 15-17 257 1999 200020-22 308 18-20 291 2499 & > 2500 342 308 * Complications include asphyxia, acidosis, hypoxia, hypothermia, meningitis, IVH, hemolysis, hypoglycemia, or signs of kernicterus. ** There is an increased risk of kernicterus. hypoalbuminemia, Hemolytic Disease of the Newborn Due to A and B Incompatibility. Major blood group incompatibility between mother and fetus usually results in milder disease than does Rh incompatibility. Maternal antibody may be formed against B cells if the mother is type A or against A cells if the mother is type B. However, usually the mother is type 0 and the infant is type A or B. Although ABO incompatibility occurs in 20-25% of pregnancies, hemolytic disease develops in only 10% of such offspring, and usually the infants are of type Ai, which is more antigenic than A2. Low antigen of the ABO factors in the fetus and newborn infant may account for the low incidence of severe ABO hemolytic disease relative to the incidence of incompatibility between the blood groups of mother and child. Although antibodies 84 against A and B factors occur without prior immunization ("natural" antibodies), there are ordinarily present in the 19S (IgM) fraction of gamma globulin, which does not cross the placenta. However, univalent, incomplete (albumin active) antibodies to A antigen may be present in the 7S (IgG) fraction, which does cross the placenta, so that A-0 isoimmune hemolytic disease may be seen in first-born infants. Mothers who have become immunized against A or B factors from a previous incompatible pregnancy also exhibit antibody in the 7S gamma globulin fraction. These "immune" antibodies are the primary mediators in ABO isoimmune disease. Clinical Manifestations. Most cases are mild, with jaundice as the only clinical manifestation. The infant is not generally affected at birth; pallor is not present and hydrops fetalis is extremely rare. Liver and spleen are not greatly enlarged, if at all. Jaundice usually appears during the first 24 hr, disappears in 3-7 days. Rarely, it may become severe, and symptoms and signs of kernicterus develop rapidly. Diagnosis. A presumptive diagnosis is based on the presence of ABO incompatibility, a weakly to moderately positive direct Coombs test, and spherocytes in the blood smear, which may suggest the presence of hereditary sherocytosis. Hyperbilirubinemia is often the only other laboratory abnormality. The hemoglobin level is usually normal but may be as low as 10-12 g/dL (100-200 g/L), reticulocytes may be increased to 10-15% with extensive polychromasia and increased numbers of nucleated red cells. In 10-20% of affected infants the unconjugated serum bilirubin level may reach 20 mg/dL or more unless phototherapy is employed. Treatment. Phototherapy may be effective in lowering serum bilirubin levels. Otherwise, treatment is directed at correcting dangerous degrees of anemia or hyperbilirubinemia by exchange transfusions with blood of the same group as that of the mother (Rh type should match the infants). The indications for this procedure are similar to those previously described for hemolytic disease due to Rh incompatibility. Phenobarbitone 5 mg/kg day orally for hasten maturation of the liver microsomal enzymes. Not useful after 7 days. Not a substitute for phototherapy. Protoporphyrin administration has been tried. Bilirubin levels decline but there is no advantage over phototherapy. It inhibits conversion of biliverdin to bilirubin by heme oxygenase. Other Forms of Hemolvtic Disease. Blood group incompatibilities other than Rh or ABO (c, E, Kell (K) end so on) account for less than 5%.of hemolytic disease of the newborn. The direct Coombs test is invariably positive, and exchange transfusion may be indicated for hyperbilirubinemia and anemia. Hemolytic disease and anemia due to anti-Kell antibodies is not predictable from the previous obstetric history, amniotic fluid OD450 bilirubin determinants, or the maternal antibody titer. Erythroid suppression may contribute to the anemia; PUBS is beneficial in actually measuring the fetal packed cell volume. Congenital infection such as cytomegalia, toxoplasmosis, rubella and syphilis, may present with hemolytic anemia, jaundice, hepatosplenomegaly, and thrombocytopenia, but the direct Coombs test is negative, and there are usually other distinguishing clinical findings. Homozygous L- thalassemia may present with severe hemolytic anemia and a clinical Figure resembling hydrops fetalis; it can be distinguished by a negative direct Coombs test and characteristic clinical and laboratory findings. Anemia and jaundice may occur in infancy from hereditary spherocytosis and, if untreated, can result 85 in kernicterus. Hemolytic anemia producing jaundice in the 1st wk of life may also be secondary to congenital deficiencies in red blood cell enzymes, such as pyruvatekinase of G-6-PD. TREATMENT OF HEMOLYTIC DISEASE Therapy is indicated when the hemoglobin and packed cell volume are low enough to compromise the oxygen-carrying capacity of the blood, which can cause congestive heart failure, respiratory distress, acidosis, poor perfusion and hypotension. The blood volume usually is normal. Therefore, the anemia is corrected by performing a partial exchange transfusion with packed red blood cells. Phototherapy. When bilirubin absorbs visible light with wavelengths of 400500nm tree types of photochemical reaction occur: photoisomerization, structural isomerization, photo-oxydation. Specific indications for the use of phototherapy are: In Rh- disease phototherapy must be start immediately. In ABO Hemolytic disease phototherapy starts if the bilirubin level exceeds 10 mg/dL (171μmol/L) at 12 hour, 12 mg/dl (205 μmol/L) at 18 hour, 15 mg/dL (256 μmol/L) at any time. Recommendations for using phototherapy in neonatal period are in the table 18. Table 18 Recommendations for using phototherapy depending of the body weight. Body weight after Instead delivery < 1500 Onset at the first 24 hours of life. Duration about 7 days 1501-1999 Without hemolysis if bilirubin 171μmol/l, if hemolysis – 136.8 μmol/l 2000 - 2499 Without hemolysis if bilirubin 205.2 μmol/l, if hemolysis – 171 μmol/l > 2500 See Figure 18 The next table shows stopping of the phototherapy (table 19) Table 19 Recommendations for the stopping of the phototherapy Body weight after delivery Bilirubin level (μmol/l) < 1000 85.5 1001-1249 102.6 1250-1499 119.7 1500-1999 136.8 2000-2499 153.9-171 > 2500 Without hemolysis – 205.2 – 256.5, if hemolysis – 205.2 Serum bilirubin levels plotted against age in term infants and preterm with erythroblastosis. Levels above the top line are predictive of an ultimate bilirubin level that will be over 20 unless the natural course is altered by treatment. Levels below the bottom line predict that the level will not eventually reach 20. These charts were developed before phototherapy was used and before the discovered of many factors that might lead to kernicterus at low bilirubin levels; however, the charts still offer guidelines to the natural progression of bilirubin levels in infants with Rh incompatibility (Figure 18, 19) Conversion of bilirubin from mg/dl to μmol/l is next: Blirubin mg/dl * 17.1 = mmol/l 86 Fig. 18,19 87 Exchange transfusion is used principally in hemolytic disease or when the bilirubin concentration is very high (see table 17). This procedure directly removes the bilirubin from the intravascular space. Unbound antibodies that initiate the hemolytic process also are removed. Indication for exchange transfusion 3. When phototherapy fails to prevent a rise in bilirubin to toxic level. 4. Correct anemia and improve congestive heart failure in hydropic infants with hemolytic disease 5. Stop hemolysis and bilirubin production by removing antibody and sensitized RBCs. 6. Immediate exchange transfusion is indicated if cord bilirubin level is over 4.5 mg/dL (77μmol/L) and the cord hemoglobin level is under 11 gm/dL (110 g/l) the bilirubin level is rising over 0.5 mg/dL (8.5 μmol/L) per hour despite phototherapy and hemoglobin level is between 11 and 13 gm/L (110-130 g/L) the bilirubin level is 20 mg/dL, or it appears that it will reach 20mg/dL (342 μmol/L) at the rate is rising there is progression of anemia but phototherapy perform 5. Repeart exchanges are done for the same indications as the initial exchanges. The exchange transfusion team should consist of two physicians, one to do the exchange and one to record its progress, and a nurse to observe the patient and provide materials. An exchange transfusion should be performed in an intensive care unit or an operating room (in emergencies, in the delivery room) with proper lighting, heating, monitoring devices, and maintenance of sterility. Route In the newborn, the routes in order of preference are: 1. Umbilical artery and vein. This allows volumetric exchange with withdrawal from the artery and infusion into the vein. The tip of the umbilical arterial catheter should be at L4 and the tip of the umbilical venous catheter should be to the diaphragm (in the inferior vena cava [IVC] or the right atrium). 2. Umbilical artery alone 3. Umbilical vein with the tip of the catheter in the inferior vena cava 4. Venous catheter introduced into the inferior or superior vena cava in the thoracic cavity via a cut down. This is necessary in some infants, especially those with omphalitis. 88 5. Peripheral arterial catheter (out) and peripheral vein catheter 6. Umbilical vein with the tip of the catheter in the portal system In older children, the preferred routes are: 1. A systemic arterial catheter (out) and a venous catheter (in) in the superior or inferior vena cava in the thoracic cavity for an isovolume exchange transfusion 2. A venous catheter in the superior or inferior vena cava in the thoracic cavity Preparation 1. Place the patient in a warm, well-lighted environment. For newborns use a radiant warmer and restrain all four extremities. Aspirate the stomach contents. Place the patient on a cardiorespiratory monitor. Have the emergency cart for CPR immediately available. 2. In all patients, before exchange transfusion measure: a. Packed cell volume and hemoglobin. b. Platelet count. c. Arterial blood-gas tensions and pH. d. Heart rate. e. Respiratory rate. f. Serum Na, K, and Ca. g. Temperature. h. Glucose level. i. Systemic arterial blood pressure. j. CVP when available. 3. In newborns with hyperbilirubinemia before exchange transfusion should also measure: a. Serum protein b. Serum bilirubin, total and direct. c. Reticulocyte count. d. Coombs test. 4. In patients undergoing exchange transfusion for endogenous (e.g., ammonia) or exogenous (e.g., carbon monoxide) toxins, measure the blood level of the toxin before and after the procedure. Method 1. In newborns with hemolytic disease use donor blood that is compatible with the mother blood. For example, if the mother is O Rh− and the infant O Rh +, use O Rh− blood so that antibodies to the Rh positive factor that remain in the infant after the exchange will not destroy donor cells. 2. Use the freshest blood possible preferably less than 1 day old. 3. In infants with thrombocytopenia (< 100,000/mm3), use fresh blood when available. 4. Warm blood to 37° C using coiled tubing .Use micro pore filter. 5. Intermittently agitate the donor unit so that the cells do not sink, leaving red blood cells-poor blood at the end of the exchange. 6. Calculate volume. Usually a two-volume exchange will remove 83% of the patient's red blood cells. 7. If the push-pull method is used use volumes of 5 ml for infants weighing less than 1500 89 gm, 10 ml for infants 1500 to 2500 gm, 15 ml for infants 2500 to 3500 gm, and 20 ml for infants over 3500 gm. 8. The exchange should not be completed in less than 60 minutes in a vigorous patient and should be slower in a patient with unstable systemic arterial blood pressure, ventilation or temperature. 9. Give 1 ml of 10% calcium gluconate for every 100 ml of ACD or CPD (citrated) donor blood when the serum calcium level is low (less than 7.5 mg/dl) at the beginning of the procedure. It is probably unnecessary to give calcium to infants who have a normal serum calcium level before exchange transfusion. 10. Observe the patient and the monitor for signs of distress. Stop the exchange transfusion if the patient's condition is unstable. 11. If the patient's condition is unstable but the exchange transfusion must proceed, measure the systemic arterial blood pressure and arterial blood-gas tensions and pH after every 100 ml exchanged. 12. After the exchange transfusion, continue to monitor the patient, especially the Dextrostix since hypoglycemia is common 20 to 60 minutes after the exchange transfusion if glucose is suddenly withdrawn. Maintain glucose until the patient is feeding again. 13. Consider giving new doses of any medications whose serum concentrations are lowered by exchange transfusions (e.g. digitalis, antibiotics and anticonvulsants). 14. If the catheters malfunction do not force blood through them. They are probably clotted, and application of force will dislodge clots, which can have disastrous results. Change the catheters immediately. 15. After the exchange transfusion measure: a. Heart rate. b. Respiratory rate. c. Temperature. d. Glucose level. e. Systemic arterial blood pressure. f. Arterial blood-gas tensions and pH. g. Packed cell volume and hemoglobin. 90 Hemorrhagic Disease of the Newborn. A moderate decrease of factors II, VII, IX and X normally occurs in all newborn infants by 48-72 hr after birth, with a gradual return to birth levels by 7-10 days of age. This transient deficiency of vitamin Independent factors probably is due to lack of free vitamin K in the mother and absence of bacterial intestinal flora normally responsible for synthesis of vitamin K. Rarely, among term infants and more frequently among premature infants there is in accentuation and prolongation of this deficiency between the 2nd and 7th days of life, resulting in spontaneous and prolonged bleeding. Breast milk is a poor source of vitamin K, and hemorrhagic complications have appeared more commonly in breast-fed than in formulated infants. This classic form of hemorrhagic disease of the newborn, which is responsive to vitamin K therapy, must be distinguished from disseminated intravascular coagulopathy and from rarer congenital deficiencies of one or more of the other factors that are unresponsive to vitamin K. Late onset (>1 wk) is often associated with vitamin K malabsorption as noted in neonatal hepatitis or biliary atresia. Types of neonatal hemorrhage: Hemorrhagic disease of the newborn due to (a) Low prothrombin level at birth due to deficiency of vitamin K. (b) Fall of prothrombin on fourth or fifth day after birth. If it falls as low as 15 -20% of normal, spontaneous hemorrhages are likely to occur, (c) Immaturity of liver for formation of vitamin K dependent factors, (d) Relatively low concentration of vitamin K in breast milk, (e) Poor endogenous intestinal production until full colonization with intestinal bacteria (from 2 to 5 day of life). 1. Those resulting from birth injures, e.g. superficial injures of scalp, intracranial and visceral hemorrhages. 2. Those due to asphyxia or infection in premature infants leading to DIC. 3. Those secondary to general or local disease, e.g. syphilis, neonatal infections and septicemia. 4. Umbilical bleeding due to mechanical cause, or after separation of the cord from an umbilical polypus, clotting factor deficiencies (especially factor XIII). 5. Thrombocytopenia due to trapping of platelets in big hematoma intrauterine and neonatal infections, isoimmune. 6. Rare blood disease, e.g. hemophilia. 7. Vaginal hemorrhage usually a manifestation of maternal hormone occurs on 5th-7th day of no consequence. 8. Swallowed maternal blood. 9. Mother on treatment with coumarin derivatives dilantin or phenobarbitone. Symptoms and Signs. This disease is characterized by bleeding that tends to be gastrointestinal, nasal, intracranial or a result of circumcision. Prodromal or warning signs (mild bleeding) may occur prior to serious intracranial hemorrhage. 1. Gastrointestinal tract - Hematemesis and melena are the most common manifestations. There is sudden onset with passage of dark tarry stool on first or 91 second day after birth. Stools that follow may consist of almost pure blood. Hematemesis occurs in about half the cases and usually follows melena. Infant soon becomes pale and collapsed and may die in a few hours or after 2 to 3 days. 2. Vagina - may be the only site of bleeding which is usually slight and continues for 23 days withdrawal bleeding-seen normally after fifth day. 3. Hematuria - usually slight loss of blood. 4. Umbilicus - severe and even fatal bleeding may occur. It often starts in the form of a steady oozing at the end of the first week. 5. Lungs - acute hemorrhagic pneumonia may be start within a few hours. Symptoms consist of rapid pallor and blood stained froth in mouth and are rapidly fatal. 6. Intracranial — in large infant is still born; if less severe — asphyxia, inability to suck well and to swallow, poor ineffective cry, twitching or convulsions, intermittent cyanosis, bulging fontanel, rigidity of limbs. Squint, nystagmus and local paralysis may be present. 7. Adrenal hemorrhage - may accompany other manifestations of anoxia at birth or later occur rarely as a part of clinical manifestation of hemorrhagic disease. 8. Skin - ecchymotic areas at points of pressure. 9. Miscellaneous - Nose, mouth, conjunctiva and retina. Laboratory Data. The prothrombin time, blood coagulation time, and partial thromboplastin time are prolonged, and the levels of prothrombin (II), and factors VII, IX, and X are significantly decreased. Vitamin K facilitates posttranscriptional carboxylation of factors II, VII, IX and X. In the absence of carboxylation such factors from PIVKA (protein induced in vitamin K absence), which is a sensitive marker for vitamin K status. Bleeding time, fibrinogen, factors V and VIII, platelets, capillary fragility, and clot retraction are normal for maturity. Management. The disease may be effectively treated with an intravenous infusion of 1-5 mg of vitamin K 1, with improvement of coagulation defects and cessation of bleeding within a few hours. However, serious bleeding, particularly in premature infants or those with liver disease, may require a transfusion of fresh frozen plasma (10 ml/kg) or fresh whole blood (20 ml/kg). The mortality rate is low among treated patients. A particularly severe form of deficiency of vitamin K-dependent coagulation factors has been reported in infants born to mothers receiving anticonvulsive medications (phenobarbital and phenytoin) during pregnancy. There may be severe bleeding with onset within the first 24 hr of life, which is usually corrected by vitamin K1, although is some the response is poor or delayed. A prothrombin time (PT) should be obtained on cord blood and the infant given 1 -2 mg of vitamin K intravenously. If the PT is greatly prolonged, and fails to improve, 10 ml/kg of fresh frozen plasma should be given. The recommended drug and dose of vitamin K (IM) has been safe and not associated with an increased risk of cancer. Although oral vitamin K (birth, discharge, 3-4 wk: 1-2 mg) has been suggested as an alternative, the oral route is not universally accepted and the IM route remains the method of choice. Vitamin K oxide - 1 mg/kg or Aqueous preparation of IM Menadione (vit-K3) thrice, repeat at 8 hourly intervals may be used. 92 Other forms of bleeding may be clinically indistinguishable from hemorrhagic disease of the newborn responsive to vitamin K but are neither prevented nor successfully treated with it. A clinical pattern identical to that of hemorrhagic disease of the newborn may also result from any of the congenital defects in blood coagulation. Hematomas, melena, and postcircumcision and umbilical cord bleeding may be present; only 5-35% of factors VIII and IX deficiencies become clinically apparent in the newborn period. Treatment of the rare congenital deficiencies of coagulation factors requires fresh frozen plasma or specific factor replacement. Disseminated intravascular coagulopathy in newborn infants results in consumption of coagulation factors and bleeding. The infants are often premature; the clinical course is frequently characterized by hypoxia, acidosis, shock, hemangiomas, or infection. Treatment is directed at correcting the primary clinical problem, such as infection, and at interrupting consumption and replacing clotting factors. The prognosis is poor regardless of therapy. Infants with central nervous system or other bleeding constitution an immediate threat to life should receive a small transfusion of fresh, compatible whole blood or plasma, as well as vitamin K, as soon as possible after blood has been drawn for coagulation studies, which should include determination of the number of platelets. The so-called swallowed blood syndrome, in which blood or bloody stools are passed, usually on the 2nd or 3 rd day of life, may be confused with hemorrhage from the gastrointestinal tract. The blood may be swallowed during delivery or from a fissure in the mother's nipple. Differentiation from gastrointestinal hemorrhage is based on the fact that the infants' blood contains mostly fetal hemoglobin, which is alkali-resistant, whereas swallowed blood from a maternal source contains adult hemoglobin, which is promptly changed to alkaline hematin upon the addition of alkali. Apt devised the following test for this differentiation: (1) Rinse a bloodstained diaper or some grossly bloody stool with a suitable amount of water to obtain a distinctly pink supernatant hemoglobin solution. (2) Centrifuge the mixture. Decant the supernatant solution. (3) To 5 parts of supernatant fluid add 1 part of 0.25 normal (1%) sodium hydroxide. Within 1-2 min a color reaction takes place: a yellow-brown color indicates that the blood is maternal in origin; a persistent pink that it is from the infant. A control test with known adult or infant blood or both is advisable. Widespread subcutaneus ecchymosis in premature infants at or immediately after birth is apparently a result of superficial blood vessels rather than of a coagulation defect. Administration of vitamine K1 to mother during labor has no effect on their incidence. Occasionally, an infant is born with petecchiae or a generalized bluish suffusion limited to the face, head, and neck, which is probably the result of venous obstruction caused by a nuchal cord or sudden increases in intrathoracic pressure during delivery. It may take 2-3 wk for such suffusions to disappear. Prevention. Administration of vitamin K to the mother in the last months of pregnancy and during labor if mother has dilantin or coumadin. Elective surgery like circumcision should be postponed till after a week, but if performed, vitamin K should be given parenteral. 93 Vitamin K 1 mg to at risk neonates at birth particularly prematures and low birth weight, forceps, LSCS and instrumental deliveries. Neonatal Thrombocytopenia. Thrombocytopenia of the newborn may indicate primary disease in the infant's hematopoietic system or may be a result of the transfer of abnormal factors from the mother. Association with Infection. Thrombocytopenia may occur in various fetal and neonatal infections and may be responsible for serious spontaneous bleeding. These include viral infections (especially rubella and cytomegalia), protozoal infections (e.g. toxoplasmosis), and syphilis and bacterial infections, especially those caused by gramnegative bacilli. Hemolysis is usually also present in infants with prominent anemia and jaundice. The liver and spleen are considerably enlarged. The bone marrow changes are variable, but reduced numbers of megakaryocytes may be seen. Immune Neonatal Thrombocytopenia. About 30% of infants born of mothers with active idiopathic thrombocytopenic purpura have thrombocytopenia resulting from the transplacental transfer of anti platelet antibodies. Rarely infants with neonatal disease have been born of mothers with past histories of FTP (but who had splenectomy) who have normal platelet counts and whose disease has been inactive for many years. Petecchiae are not present initially but appear in a generalized distribution within a few minutes after birth. Bleeding from the bowel or kidney and intracranial hemorrhage may occur. In mild cases may be present abnormal findings. Hepatosplenomegaly is not present. The duration of the thrombocytopenia is 2-3 month. Therapy is not strikingly successful, but intravenous immunogldbulin, exchange transfusions, or platelet transfusions may be of temporary value in arresting acute bleeding. Corticosteroid therapy has not been proved beneficial. Because of the self-limited nature of the disease, splenectomy is contraindicated. Corticosteroid therapy given to the mother 1 wk prior to delivery or administration of intravenous gamma globulin to the mother late in pregnancy may reduce the severity of the disease in the mother and perhaps in the infant. When the fetus has platelet antigens that the mother does not have alloimmunization may occur. If maternal antibodies to fetal platelet antigens reach a sufficiently high titer, enough may cross the placenta to produce thrombocytopenia in the fetus. The disease may be familiar and first-born infants are frequently affected. Clinical signs include petechiae and other hemorrhagic manifestations. Anti platelet antibodies can be demonstrated in about 50% of cases using sensitive tests. The PLA-1 antigen is most frequently involved. Infants born to mothers with anti platelet alloantibodies are the most severely affected. Exchange transfusion is temporarily effective is stopping bleeding. Intravenous gamma globulin given to the affected newborn may be helpful. If compatible platelets can be obtained (these are most easily procured by preparing washed platelet concentrates from the mother), they after specific, effective therapy. Infants born of successive pregnancies may be affected. Elective cesarean section has been advocated to spare the infant's head the trauma of delivery percutaneous umbilical blood sampling will 94 diagnose fetal thrombocytopenia and permits fetal platelet transfusions with maternal platelets. When the mother has drug-induced thrombocytopenia, both antibody and drug may cross the placenta and cause neonatal thrombocytopenia. Corticosteroid therapy, and especially exchange transfusions, should be considered when bleeding manifestations and severe. Congenital Thrombocytopenias. 1.Wiskott-Aldrich syndrome. WAS consists of eczema, thrombocytopenic hemorrhage, and increased susceptibility to infection because of an immunologic defect that is transmitted as an X-linked recessive trait. The bone marrow contains a normal number of megakaryocytes, but many have bizarre nuclear morphology. Homologous platelets survive normally when transfused into these patients, but autologous platelets have a shortened life span and are small in size. WAS may represent an unusual circumstance in which thrombocytopenia results from abnormal platelet formation or release, despite quantitatively adequate numbers of megakaryocytes. Splenectomy has often been followed by overwhelming sepsis and death, but significant improvement in thrombocytopenia occurs after splenectomy. Prophylactic use of penicillin is essential post splenectomy. About 5% of patients with WAS develop lymphoreticular malignancies. A few cases have been reported to benefit from the administration of transfer factor or from bone marrow transplantation. Other Inherited Thrombocytopenias. Other types of inherited thrombocytopenias have been described. Some are X linked and some have autosomal transmission. Responses to therapy, including splenectomy, have usually been disappointing. The inordinately high mortality of young males with splenectomy for presumed idiopathic thrombocytopenic purpura (ITP) suggests that, even without other stigmata. X-linked thrombocytopenia may represent a variant of WAS. Thus, the young thrombocytopenic male must be carefully studied before a diagnosis of ITR mode. A platelet survival study may be indicated in such patients. Thrombopoietin Deficiency. A few patients have hade chronic thrombocytopenia attributed to deficiency of a megakaryocyte maturation factor contained in normal plasma. Plasma infusions repeatedly produced a sustained rise in the platelet count. In somewhat similar cases, episodic thrombocytopenia and microangiopathic hemolysis were reversed by infusions of plasma. Thrombocytopenia with Cavernous Hemangioma. (Kasabosh-Merrit Syndrome) Some infants with large, cavernous hemangiomas of the trunk, extremities, or abdominal viscera have severe thrombocytopenia and other evidence of intravascular coagulation. Histological and isotopic studies indicate that platelets are trapped and destroyed within the extensive vascular bed of the tumor. The peripheral blood reveals 95 thrombocytopenia and red blood cell fragments, and the bone marrow contains adequate numbers of megakaryocytes. Spontaneous thrombosis within the tumor may lead to obliteration of the vascular channels and spontaneous recovery; radiation therapy in a single dose of 600-800 rad may accelerate this process, but repeated courses may be necessary. When anatomically feasible, external compression or total excision may be attempted, but surgery can be associated with uncontrollable hemorrhage. Corticosteroids and interferon may hasten involution and warrant trial, especially in the young infant. Splenectomy is contraindicated. Congenital Hypoplastic Thrombocytopenia with associated Malformations. (Thrombocytopenia Absent Radius [TAR] Syndrome). Severe thrombocytopenia associated with aplasia of radii and thumbs, and with cardiac and renal anomalies, occurs as a familial condition. Severe hemorrhagic manifestations are evident in the first days of life. Hemoglobin levels are normal; leukocytosis and even leukemond reactions have been found in some patients. Megakaryocytosis are absent from the bone marrow. The anomalies in this disease are similar to those observed in Fanconi syndrome. No infants with congenital hypoplastic thrombocytopenia have been reported to develop full-blown Fanconi syndrome, nor have both conditions been observed in the same family. Consumption Coagulopathy. (Disseminated Intravascular Coagulation Syndromes - DIC). Consumption coagulopathy refers to a large group of conditions, including disseminated intravascular coagulation (DIC). Consequences of this process include widespread intravascular deposition of fibrin, which may lead to tissue ischemia and necrosis, a generalized hemorrhagic state, and hemolytic anemia. Etiology. A number of pathologic process may incite episodes of DIC, including hypoxia, acidosis, tissue necrosis, shock, and endothelial damage (Fig. 4). Accordingly, it is not surprising that a large number of diseases have been reported to be associated with DIC, including incompatible blood transfusions, septic-shock (especially gram-negative), rickettsial infections, snakebite, purpura fulminous, giant hemangioma, malignancies, and acute promyelocytic leukemia. Clinical Manifestations.Most frequently DIC accompanies a severe systemic disease process. Bleeding frequently occurs from sites of puncture of vein or surgical incision, with associated petechiae and ecchymoses. Tissue thrombosis may involve many organs and can-be most spectacular as infarction of large areas of skin and subcutaneous tissue or of kidneys. Anemia caused by hemolysis may develop rapidly. Laboratory Findings. There is no well-defined sequence of events. The consumption coagulation factors (II, V, VIII, and fibrinogen) and platelets may be consumed by the ongoing intravascular clotting process, with prolongation of the prothrombin, partial thromboplastin and thrombin times. Platelet counts may be profoundly depressed. The blood contains fragmented burr and helmet-shaped red blood cells (schizocytes), changes referred to as microangiopathic. In addition, because the fibrinolytic 96 mechanism is activated, fibrin split products (FSP) appear in the blood. Table 16 presents the laboratory findings in children with the three common acquired coagulation defects. Table 20 Laboratory Findings in Disseminated Intravascular Coagulation, Vitamin K Deficiency, and Disorder Liver Disease. DIC Vitam Liver Deficie in K Disease ncyP PTT P P PT P P P TT P N P Platelet L N N/L count ESPs + +Fibrinogen L N L Factor VIII L N N/I DIC - disseminated intravascular coagulation; PTT - partial thromboplastin time; PT - prothrombine time; TT - thrombine time; ESPs - fibrinolytic split products; P - prolonged; N - normal; L - low; N/L - normal or low; + - present; + - -present or absent; I - increased. The D-dimmer assay is equally sensitive and more specific for DIC than the fibrin degradation product (FDP) test. D-miter is a neo-antigen formed following the thrombin - initiated generation of fibrin from fibrinogen, followed by cross - linking of fibrin by factor VIII and plasmin digestion of the cross - linked fibrin. Treatment. The most important component of therapy is control or reversal of the process that initiated the DIC. Infection, shock, acidosis, and hypoxia must be treated promptly and vigorously. If the underlying problem can be controlled bleeding quickly ceases, and there is improvement of the abnormal laboratory findings. Blood components are used for replacement therapy in patients who have hemorrhage. This may consist of platelet infusions (for thrombocytopenia), cryoprecipitates (for hypofibrinogenemia), and/or fresh frozen plasma (for replacement of other coagulation factors and natural inhibitors). In some patients the treatment of the primary disease may be inadequate or incomplete, or the replacement therapy may not be effective in controlling the hemorrhage. When this occurs the DIC may be treated with anticoagulants to prevent ongoing consumption of factors. Heparin is the drug of choice and can be administered on an intermittent or continuous intravenous treatment schedule. Using the intermittent intravenous schedule, heparin is given in a dose of 75-100 units/kg every 4 hr. With the 97 continuous schedule, 50-75 units of heparin/kg is given as a bolus followed by a continuous infusion of 15-25 units/kg/hr. The duration and effectiveness of heparin therapy can be judged by serial measurements of the platelet count and plasma fibrinogen concentration. Heparin has been found to be an affective drug in children with DIC associated with purpura fulminans and promyelocytic leukemia. Lower doses (10-15 units/kg/hr without a loading dose) are used for those with progranulocytic leukemia. Heparin is not indicated and has been reported to be ineffective in septic shock, heat stroke, massive head injury, and incompatible blood transfusion reaction. 98 Supplement TESTS 1. What cause of asphyxia is wrong A.knotting of cord B.maternal staphylococcus C.breach presentation D.maternal acute bleeding 2. Asphyxia is severe if Apgar scores are A.0-3 B. 0-4 C. 1-4 D. 4-6 3. Assessment of newborn by Apgar should be perfomed at the A. 1 and 5 minutes of life B. 1 and 10 minutes of life C. 5 and 10 minutes of life D. 1 and 20 minutes of life 4. Step A resuscitation includes A. clearing the nose then the mouth B. clearing the mouth then the nose C. intubations D. clearing the mouth then the nose with tactile stimulation 5. Step C resuscitation includes A. introduction of epinephrine B. cardiac compression C. assessment of respiration D. cardiac compression and ventilation 6. Cardiac compression should be made if a heart rate is A. 80 B. 100 C. 60 D. 90 7. What kind of clinical sings must be assessed by Apgar A. pulse, respiration, photoreaction, arterial pressure B. pulse, temperature, reflexes, arterial pressure C. pulse, respiration, muscular tone, skin, reflexes D. pulse, respiration, photoreaction, muscular tone, skin 8. For assessment of stage of HIE the following scale should be used A. Apgar B. Sarnat C. Ballard D. Downess 9. Development of neonatal seizures excludes A. metabolic causes B. IVH C. LBW D. meningitis 10. Normal level of cord hematocrit in the neonate A. 61 B. 53 C. 45 D. 57 11. Dose of 10% Calcium gluconate for treatment of hypocalcemia A. 2 mg/kg B. 1 mg/kg C. 10 mg/kg D. 5 mg/kg 99 12. Diagnosis of neonatal hypoglycemia if a blood glucose level is less than A. 1.7 mmol/l B. 2.2 mmol/l C. 3.3 mmol/l D. 5.5 mmol/l 13. Predisposing factors of IVH, exclude A. premature B. pneumothorax C. conjunctivitis D. RDS 14. The only possible complication of IVH A. pneumonia B. hydrocephalus C. absent of vision D. conjunctivitis 15. The infant from pregnancy of 38 week’s gestation has body weight 2400 gr. The right statement is: A. term with normal body weight B. premature but appropriate size for gestational age C. premature but with weight small for gestational age D. term but small for gestational age 16. Preterm infant has no respiration. During first seconds of life the following is necessary A. clearing of airways B. ventilation of Ambu bag C. intubation and ventilation D. Apgar assessment 17. For assessment of neonatal maturity the following scale should be used A. Apgar B. Sarnat C. Ballard D. Downess 18. Health preterm infant needs the following calories for maintenance of body weight during first day of life A.100 kcal/kg/day B.50 kcal/kg/day C.120 kcal/kg/day D.140 kcal/kg/day 19. An infant is preterm if he weight or age is A. less than 2500 g B. less than 37 week’s gestation C. less than 2500 g and less than 37 week’s gestation D. less than 38 week’s gestation 20. Initial dose of glucose for parenteral nutrition of preterm is A. 10-15 g/kg/24 hr B. 15-20 g/kg/24 hr C. 25-30 g/kg/24 hr D. 5-10 g/kg/24 hr 21. Surfactant synthesis starts at A. 12 weeks of gestation B. 20 weeks of gestation C. 22-24 weeks of gestation D. 30-32 weeks of gestation 22. Factor decreasing surfactant synthesis which is wrong A. cold stress B. hypoglycemia C. acute hypoxia D. prolonged rupture of membrane 100 23. Nondependent surfactant disease excludes A. choanal atresia B. tracheoesophagal fistula C. primary atelectasis D. transient tachypnea of the newborn 24. Basic substance of the surfactant is A. protein B. water C. carbohydrates D. lipids 25. Lecithin sphingomyelin ratio for lung maturity composes A. 0.5 B. 1.0 C. 2.0 D. 1.5 26. For assessment of respiratory insufficiency the following scale should be used A. Apgar B. Sarnat C. Ballard D. Downess 27. Complications of HMD exclude A. HIE B. persistence of ductus arteriosus C. IVH D. anemia 28. Surfactant introduction should be performed A. intravenously B. intramuscularly C. orally D. endotracheally 29. Factor stimulating surfactant production A. ACTP B. estriol C. thyroxin D. progesterone 30. Sepsis may be in infant excludes A. premature B. rupture of membrane > 24 hr C. conjunctivitis D. macrosomia 31. Laboratory evaluation of the neonatal sepsis includes A. EEG B. ECG C. C-reactive protein D. ά-fetoprotein 32. Dose of ampicillin for newborn 0-4 days of life is A. 50 mg/kg/day B. 100 mg/kg/day C. 150 mg/kg/day D. 200 mg/kg/day 33. The term “TORCH” includes a number of infections except A. rubella B. staphylococcus C. cytomegalovirus D. syphilis 34. Optimal drug for treatment of CMV infection is A. Gancyclovir B. immunoglobulin C. interferon D. penicillin 35. Prevention of congenital herpes if a women has genital herpes before delivery consists in A. interferon for mother B. interferon for newborn C. caesarian section D. Gancyclovir for mother 36. Classic triad of congenital rubella consists of 101 A. congenital heart disease, eyes pathology, deafness B. rash, meningitis, pathology of CNS C. congenital heart disease, rash, pathology of CNS D. deafness, rash, pathology of CNS 37. Classic triad of congenital toxoplasmosis consists of A. hydrocephalus, chorioretinitis, brain calcification B. deafness, chorioretinitis, brain calcification C. congenital heart disease, rash, microcephalia D. deafness, rash, pathology of CNS 38. Treatment of congenital syphilis includes A. penicillin 100 U/kg B. ampicillin 50 U/kg C. penicillin 50U/kg D. ampiox 100 U/kg 39. The child 15 days old has chronic conjunctivitis. What probable agent may cause this condition? A. staphylococcus B. Streptococcus C. chlamidia D. fungi 40. Normal level of cord bilirubin is A. 8.5 μmol/l B. 34 μmol/l C. 102 μmol/l D. 137 μmol/l 41. The cause of the HMD is A. deficiency of surfactant B. deficiency of corticosteroids C. intrauterine pneumonia D. apnea 42. Prescribe the medicine to a mother for prevention of HMD A. prednisolon B. betamethasone C. dexamethasone D. hydrocortisone 43. The cause of Hemolytic Disease of newborn is A. fetomaternal blood group incompatibility B. red-cell enzyme deficiency C. CliglerNajar syndrome D. sepsis 44. Rh-Hemolytic Disease of newborn may manifests as, except A. hemolytic anemia B. hydrops fetalis C. jaundice D. hypoglycemia 45. Hemolytic Disease of newborn occurs if A. maternal blood Rh+, fetus blood Rh- B. maternal blood Rh-, fetus blood RhC. maternal blood Rh-, fetus blood Rh+ D. maternal blood Rh+, fetus blood Rh+ 46. Hemolytic Disease of newborn occurs if A. maternal blood 0 (I), fetus blood A (II) B. maternal blood A (II), fetus blood A (II) C. maternal blood 0 (I), fetus blood 0 (I) D. maternal blood A (II), fetus blood 0 (I) 102 47. SUN DOWN (SET) symptom is present in the newborn with A. jaundice B. kernicterus C. acidosis D. hyperosmolality 48. Normal level of hemoglobin in the newborn is A. 120-140 B. 100-140 C. 140-160 D. 180-210 49. Indication for exchange transfusion is A. cord bilirubin level 77 μmol/l, Hb 110g/l B. cord bilirubin level 51 μmol/l, Hb 180g/l C. cord bilirubin level 51 μmol/l, its reasing 3.1 μmol/l hr D. cord bilirubin level 51 μmol/l, Hb 170g/l 50. Vitamin K dependent coagulation factors are A. II, V, X, XI B. II, VII, IX, X C. II, VII, VIII, X D. II, VII, VIII, IX ANSWERS: 1.B. 2.A. 3.A. 4.B. 5.D. 6.C. 7.C. 8.B. 9.C. 10.B. 11.A. 12.B. 13.C. 14.B. 15.D. 16.A. 17.C. 18.B. 19.B. 20.A. 21.C. 22.D 23.C. 24. D. 25.C. 26.D. 27.D. 28.D. 29.C. 30.D. 31.C. 32.A. 33.B. 34.A. 35.C. 36.A. 37.A. 38.A. 39.C. 40.B. 41.A. 42.B. 43.A. 44.D. 45.C. 46.A. 47.B. 48.D. 49.A. 50.B. 103 LITERATURE 1. Nelson Textbook of Pediatrics, ed. 16th. 2000. 2. O.P. Ghai. Essential Pediatrics. ED 4th 3. M Secar The pocket Pediatrician Cembridge Universitti Press. 1996. 4. Manual of Neonatal Care. Forth Edition. Ed. John P.Cloherty,M.D., Ann R Stark,M.D. Lippincott-Raven. Philadelphia*New York - 768p. 5. Г.М.Траверсе, С.М. Цвиренко, Т.И.Мизгина. Жовтяниці у новонароджених. Навч.методич. Посібник.Полтава „Верстак” 2003.-87с. 6. Практичний посібник з неонатології. :6-те видання. За редакцією Судакара Г. Езутачана, M.d., D.C.H., Ph.D., Дмитра О.Добрянського, M.D., Ph.D/. Львів-Детройт. 104