Heat capacity of a metal
advertisement

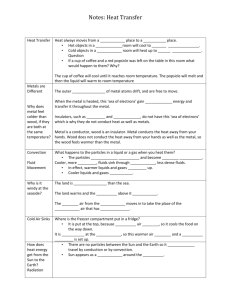
Chapter 12 lab investigationFinding the Specific Heat of a Metal Problem: Determine the specific heat of at least two different metals. Compare your results with the accepted values of these metals, which you can find on line Background: The “specific heat capacity” of a material is defined as the heat required to raise a unit mass (1 gram) of the substance by a fixed temperature (1 oC). This is sometimes also called the “specific heat” of a material, and is often abbreviated “Cp” (which stands for heat Capacity at a constant pressure). As we have already covered in class, the heat capacity of water is exactly 1 cal/(g oC). It takes 1.00 calorie, to raise the temperature of 1.00 gram of water 1.00 oC. This is how the calorie is defined. If needed, you can convert calories to Joules with the conversion factor 1.000 cal = 4.184 Joules The specific heat capacity of water is a constant value, but other materials (like metals) have their own constant heat capacity. In this lab, you will be measuring and comparing the heat capacity of two metals. Work in groups of two to complete this activity. The formula for heat change in a material is q=m T Cp (In words, this means: heat absorbed (q) = mass (m) * temperature change (T)* specific heat(Cp) all four variables must refer to the same material- for example, the heat absorbed by a sample of water equals the mass of water, times change in temperature of the water, times specific heat of the water (which is always 1 cal/g oC for water). When a heated metal cube transfers its heat to a sample of water, in a perfectly insulated container, the heat absorbed by the water must be exactly equal to the heat given off by the metal cube. There is a perfect transfer of energy from the cube to the water. q(water) = - q(metal). You can use this relationship to determine the heat capacity of a metal. Materials (per group) hot plate 250 or 500 mL glass beaker for heating water metal cube enough water to cover the metal cube when it is placed gently at the bottom of the glass beaker. Styrofoam cup, placed inside a plastic beaker for stability and insulation 100 mL room temperature water in the Styrofoam cup. one or two thermometers. 100 mL volumetric cylinder Tongs. Procedure: 1. Measure 100 mL (exactly) of room temperature water in the volumetric cylinder. Use the known density of water to determine the mass of this water. Pour the water into the Styrofoam cup. Record the temperature of this water at room temperature. This is your starting temperature for the water. 2. Choose one of the metal cubes. Record what type of metal it is (copper, iron, brass, or aluminum). And determine its weight. 3. Place the metal cube carefully in the bottom of the glass beaker. Don’t break the beaker. Fill the beaker with enough tap water to cover the metal cube. Heat the beaker and its contents on the hot plate, until it approaches boiling. 4. Just before taking the metal cube out of the boiling water, measure the temperature of the hot water. You can treat this measurement as the starting temperature of the metal block, since you have no other means to measure the internal temperature of a solid block. 5. Take the metal cube out of the hot water, and transfer it immediately to the foam cup. Stir the water around the metal cube in the foam cup with the thermometer, until the thermometer reading becomes stable. You can treat this measurement as the final temperature of both the water and the final temperature of the metal block. 6. Calculate the change in temperature of the water in the foam cup. Show your work. Label this T (H2O) 7. Calculate the heat absorbed by the water in the foam cup. Use the equation q=m T Cp All variables must apply to the water in the cup. Label the solution to this equation “q (water)” 8. Based on the heat absorbed by the water, how much heat was emitted by the metal cube? Label this value “q (metal)” 9. Calculate the change in temperature of the metal block label this T (metal) 10. Now solve for the specific heat of the metal. Again use the equation, q=m T Cp but this time make sure all the variables apply to the metal. You took the mass of the metal, calculated the change in temperature of the metal, and calculate the heat absorbed by the metal. The only unknown to solve for is Cp, the specific heat of the metal. 11. Choose a second metal cube, and repeat the lab. If you finish early, try a third. Report: Show all measurements and all calculations from the procedure in a neatly formatted report. Your measurements and calculations must be handwritten. Be sure to pay proper attention to units, dimensional analysis, and significant figures. Go on line, and find the accepted value for the specific heat of your metals. If the value posted on line is not written in the same units you calculated, show the necessary conversions to make it the same units. Calculate the %error in your experiment. In a typed paragraph, discuss the possible sources of error in this experiment (focusing especially in issues related to experimental design). Describe practical changes to the experimental design which could correct for these problems. Use vocabulary like “heat transfer” and “specific heat” (and related chapter vocabulary) in your discussion to show that you understand their meaning.