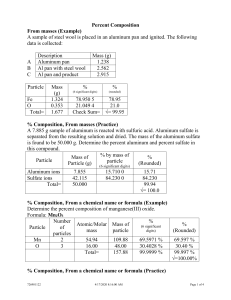
Li-chem.1a.sample.1
advertisement

College Chemistry 210 MT#1 (100 pts) 1. A sample of 24.0 g of ozone, O3, contains the same number of atoms as (a) 58.7 g of nickel (b) 27.0 g of aluminum (c) 38.0 g of fluorine (d) 1.51 g of hydrogen (e) 32.0 g of oxygen 2. What is the percentage of mass of oxygen in vanillin, C8H8O3, which is used as a flavoring? 3. C3H8 + O2 CO2 + H2O (unbalanced) How many grams of oxygen are required to burn 18.4 grams of C3H8? 4. If 100.0 g of O2 are mixed with 100.0 g of H2 and the mixture is ignited, what mass of water is produced? 5. How many electrons does the ion 60 Co3+ have? 6. An element X has the following isotopic composition: X-200, 90%, X-199,8.0% and X-202, 2.0%. Its atomic mass is closest to 7. The formula for aluminum sulfate is 8. Choose the name-formula pair that does not match. (a) ammonium carbonate (NH4)2CO3 (b) calcium hydrate CaH2 (c) sodium chlorate NaClO3 (d) perchloric acid HClO4 (e) potassium hydroxide KOH 9. The area of a 16 in. pizza is 201 in2, express this area in square centimeters (given: 1 in = 2.54 cm) (a) 31.2 (b) 125 (c) 511 (d) 1.30 x 103 (e) 5.19 x 103 10. How many iron ions (Fe3+) are present in 166.6 g FeCl3? 11. The density of carbon tetrachloride is 1.59 g/mL. How many moles are present in 80.0 mL of carbon tetrachloride? 12. Aluminum reacts with oxygen to produce aluminum oxide. If 6.0 moles of Al reacts with excess O2, how many moles of Al2O3 can be formed? 13. A mixture of MgCO3 and MgCO3.3H2O has a mass of 3.883 g. After heating to drive off all the water the mass is 2.927 g. What is the mass percent of MgCO3.3H2O in the mixture? 14. When 1.500 g of a metal oxide is heated, the compound decomposes in the reaction shown below. 2 M2O(s) 4 M (s) + O2(g) If 0.1035 g O2 is produced, what is the identity of the metal? 15. When a solution of lithium chloride and a solution of ammonium sulfate are mixed, (a) a precipitate forms (b) a new salt is formed (c) a gas is evolved (d) an acid and a base are formed (e) no reaction occurs 16. A precipitation is expected when an aqueous solution of potassium iodide is added to an aqueous solution of (a) sodium sulfate (b) iron(II) chloride (c) calcium perchlorate (d) lead (II) nitrate (e) barium hydroxide 17. Which of the following is a weak electrolyte in aqueous solution? (a) H2SO4 (b) HI (c) HNO3 (d) H2S (e) HCl 18. For the reaction Pb(s) + PbO2(s) + 2 H+(aq) + 2HSO4-(aq) 2PbSO4(s) + 2H2O(l) The oxidizing agent ( be specific) is (a) Pb (b) PbO2 (c) H+ (d) HSO4(e) PbSO4 19. How much 0.54 M NaCl solution can be prepared via the dilution of 120. mL of a 12.0 M NaCl solution? 20. Calculate the molarity of the solution that contains 100.0 g of NaOH in 200. mL of solution