physical geology-vocab
advertisement

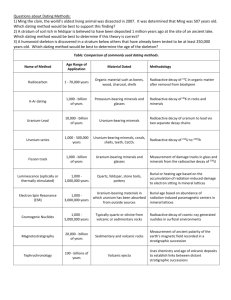
!GLG 101-Illustrated Vocabulary-Chapter 8 !Geologic Time copyright-Roger Weller-2003 !absolute dating *any method of gelogic age dating in which the age of the material is expressed in a number of years. !alpha decay *a form of radioactivity in which a helium-4 nucleus is ejected from an atomic nucleus; the least penetrating form of radioactivity. !alpha particle *a helium-4 nucleus consisting of two protons and two neutrons !angular unconformity *a structural arrangement of inclined sedimentary beds than been eroded, forming an erosion surface upon which a new set of sedimentary beds have been deposited parallel to the erosion surface. !beta decay *a neutron inside of an unstable isotope decays into a proton, neutrino, and electron; the proton stays within the nucleus, while the electron and neutrino are ejected at great speeds. !beta particle *the high-speed electron ejected from a radioactive atomic nucleus undergoing beta decay !carbon 14 dating *a radiometric age dating process in which the age of an organic sample is determined by a comparison of radioactive carbon-14 in the sample compared to total carbon content. !daughter product *the isotope left behind when a radioactive isotope undergoes radioactive decay !fission track dating *cosmic rays or radioactivity produce long trails of damaged compounds in minerals. Certain materials, when chemically treated, reveal these trails which are called fission tracks.. The older the material the more trails will show up. The age of the material is proportional to the density of fission tracks. !gamma ray *the most penetrating form of radioactivity; a high-energy X-ray is ejected from the atomic nucleus. !geochronology *the science of determining the age of geologic materials and events !isotope *a specific variety of a chemical element, distinguished by its atomic mass number. !parent element *the original unstable isotope that will undergo radioactive decay !potassium-argon dating *a method of radioactive age dating in which the amount of argon-40 in the rock in compared to the total potassium content. Argon-40 is produced by the decay of potassium-40, a radioactive istope of potassium that is found in all minerals that contain potassium. !principle of cross-cutting relationships *a fracture, fault, or dike that cuts across another structure is younger than the structure it crosses. !principle of lateral continuity *water-lain sediments are deposited in a continuous layer that stops either bcause of encountering a barrier or because of running out of material. !principle of original horizontality *water-lain sediments are almost always deposited on a nearly horizontal surface. !principle of superposition *in an undisturbed sequence of water-lain sedimentary beds, the oldest layer is at the bottom and the youngest is at the top. !principle of uniformitarianism *the laws of chemistry and physics observed today are the same as those in the past. !relative dating (not a reference to Arkansas cultural patterns) *The age of a geologic material is placed within a sequence of events; a specific age in years is not given. !radioactive decay *the process by which an atomic nucleus suddenly emits an alpha or beta ray, resulting in a change in the number of protons and neutrons which then changes the atomic number of the atom. The loss of a beta particle increases the atomic number by one and the loss of an alpha particle decreases the atomic number by 2 and the atomic mass number by 4. !radioactive half life *the amount of time it takes for half of the atoms of a specific radioactive isotope to decay !radioactivity *the spontaneous decay of an atomic nucleus. !strontium-rubidium dating *a radiometric dating process in which the age of a geologic material is determined by the ratio of the daughter product of strontium-87 to the amount of the parent isotope, rubidium. !uranium-lead dating *one of the very first techniques for determing the absolute geologic age of a material. Both uranium-235 and uranium-238 are radioactive isotopes of uranium. In the radioactive decay process, uranium-235 eventually turns into lead-207 and uranium-238 turns into lead 206. By determining the ratio of the parent istopes to their daughter products, the age of the sample can be determined.