Open
advertisement

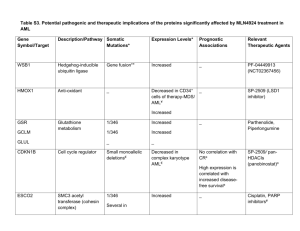
[P0673] CLOFARABINE AS A SALVAGE THERAPY AND A BRIDGE TO TRANSPLANTATION IN PATIENTS WITH HIGH RISK ACUTE LEUKEMIA AND RELEVANT COMORBIDITY. A SINGLE CENTRE EXPERICENCE S Imbergamo1, G Binotto2, C Gurrieri2, T Berno2, F Piazza2, M Ermani2, R Zambello2, G Semenzato2. 1University of Padua, Padova, Italy; 2University of Padua, Padua, Italy Backgroud. Acute myeloid leukemia (AML) is an aggressive hematological malignancy and the outcome of patients with relapsed/refractory disease still remains unsatisfactory. Clofarabine is a second generation nucleoside analog with efficacy in acute leukemia. Combination therapies of Clofarabine either with cytarabine arabinoside (ARA-C) in AML or with cyclophosfamide in acute lymphoblastic leukemia (ALL) are feasible and effective as induction therapy or salvage treatment. Aims. To determine the efficacy and safety of clofarabine as a salvage therapy and as a bridge to transplantation in frail patients with unfavourable cytogenetics relapsed/refractory acute leukemia. Methods. A retrospective analysis was conducted on 21 patients with acute leukemias treated in our Department. The diagnosis was made according to 2008 WHO criteria. Median age at diagnosis was 52 years (range 19-76); 12 (57%) patients with relapsed and 9 (43%) with refractory acute leukemias; AML (n: 19)/ALL (n: 2); patients were treated with a median of 2 prior regimens (range 1-5). Fifteen patients (71.4%) were considered at high, one intermediate and five (23.8%) low cytogenetic risk. Eighteen patients (86%) had significant comorbidity (48% had high risk Charlson score index). AML patients received salvage therapy with ARA-C 1 g/m2 for 5 day and Clofarabine, 40 mg/m2 for 5 days, from day 2 to day 6, whereas in ALL patients Clofarabine was combined with Cyclophosfamide 400 mg/m2, given for 5 days. Results. Seven patients (33%) achieved complete remission (CR); among these, 3 (14%) were classified as high risk cytogenetic at the start of therapy. The median DFS was 110.5±179 days (median: 30; range: 3 - 740), while the EFS was 36.8±39.8 (median: 21; range: 3-150). Neither the DFS nor EFS were significantly associated to cytogenetic risk profile (p=0.96 e p=0.33, respectively). The DFS was correlated with EFS (Rho=0.61, p=0.003). Eight patients (38%), three in complete remission and three with persistence of disease, underwent transplantation. Two cases relapsed after allogeneic bone marrow transplantation (allo-BMT) and were treated with clofarabine as salvage therapy; the median survival from the beginning of therapy was 100 days (95% CI: 35-165). The median survival was significantly correlated with transplantation (log rank, p=0.03), resulting 157 days (95% CI: 86-228) in transplanted patients versus 58 days (95% CI: 0-134) in non transplanted patients. Side effects with clofarabine included nausea (49%), transient liver dysfunction (71%), skin rashes (43%), mucositis (31%). Patients were treated with antibiotic prophylaxis with fluoroquinolones. We documented 13 (61%) febrile neutropenia, 8 (38%) septic shock, 5 (23%) cardiac abnormalities (3 atrial fibrillation and 2 heart failure). Conclusion. Clofarabine in combination with ARA-C or Cyclophosphamide represents a promising combination in the treatment of acute leukemias both as a salvage therapy to induce remission and as a bridge to transplant. [P1222] CLOFARABINE IN COMBINATION WITH CYTARABINE (ARA-C) FOR TREATMENT OF RELAPSED/REFRACTORY ACUTE MYELOID LEUKEMIA IN ADULT PATIENTS A Malato1, A Santoro1, S Magrin1, R Felice1, D Turri1, R Di Bella1, D Salemi1, F Acquaviva1, R Scimè1, F Fabbiano1. 1Ospedali Riuniti Villa Sofia-Cervello, U.O. di Ematologia e UTMO, Palermo, Italy Introduction. Relapsed/refractory AML patients have a poor prognosis, with CR rates of 1%30%, unless allogeneic hematopoietic stem cell transplantation (HSCT) is an available option. Although retrospective modeling studies have demonstrated the prognostic value of selected parameters, responses with salvage therapies remain still poor. It was previously established the activity of clofarabine plus cytarabine in AML relapse (clofarabine dosed once daily for 5 days with 40 mg/m2 followed 4 hours later by ara-C at 1 g/m2 per day). However, modifications of this combination in AML therapy of relapsed/refractory patients warrant further evaluation. Aim. To determine the efficacy and safety of clofarabine and cytarabine (Ara-C) in adult patients with relapsed or refractory acute myeloid leukemia (AML). Methods. Patients aged 35-66 years with refractory/relapsed AML were treated at the dose of clofarabine 30 mg/mq on days 1-5 + cytarabine 1000 mg/mq gg on days 1-5. We evaluated the complete remission rate (CRR), duration of remission (DOR) and overall survival (OS). Minimal residual disease (MRD) by molecular targeting was considered in all patients. Results. Seventeen patients received clofarabine 30 mg/mq on days 1-5 + cytarabine 1000 mg/mq gg on days 1-5 (their characteristics are summarized in Table 1), followed by gentuzumab therapy in only three patients. All patients had relapsed/refractory myeloid leukemia and had received multiple priors tharapies. Two pts had received a prior hematopoietic stem cell transplant (HSCT). Eight patients achieved a morphologic complete remission (CR);fourpatients went on to receive allogeneic transplants after clofarabine/ARA-C salvage. The complete remission rate (CRR) was 52,94%. The Median of Overall survival for all patients was 59 days (range 23-769), while the media of Overall survival (OS) was 158,41 days, and we estimated a duration of remission (DOR) as101,50 days in median (range 3-785), and 260 days in media (we calculated from the first day of remission). Treatment was complicated by neutropenic fever (n = 9), grade III-IV mucositis (n = 2), skin rush (n = 2) grade II- III, hepatic transaminase elevations(n = 1). Two patient died of sepsis during the induction. Conclusions. Combination treatment with clofarabine 30 mg/mq and ARAC 1000 mg/mq in adults pts with refractory or relapsed AML resulted in an ORR of 52,94 %, and of the 8 patients who achieved a CR, four (50%) proceeded to HSCT (two are still alive and in complete remission). The safety profile is acceptable in this relapsed/refractory population, and our results are very similar to previous regimes using higher clofarabine dosages. More studies with this combination in adults are warranted. [P0072] ORAL CLOFARABINE PLUS LOW-DOSE CYTARABINE IN PREVIOUSLY TREATED AML AND HIGH-RISK MDS PATIENTS = 60 YEARS OF AGE M Pagel1, R Sandhu2, T Gooley2, B Scott2, K Shannon-Dorcy3, C Dean2, R Appelbaum2, H Estey2. 1Fred Hutchinson Cancer Research Center, Seattle, WA, United States of America; 2FHCRC, Seattle, WA, United States of America; 3FHCRC, Seattle, United States of America Background. For many older patients with AML or high-risk MDS, the risks of standard induction chemotherapy may outweigh the benefits. The combination of intravenous (IV) clofarabine and cytarabine (ara-C) appears well tolerated and active in older or relapsed adults with AML. A randomized trial in untreated patients age ≥60 suggested that IV clofarabine plus low-dose ara-C (LDAC) was more effective than clofarabine alone. Oral clofarabine offers advantages to older patients and a phase II trial in higher-risk MDS patients confirmed the drug's activity, leading us to combine oral clofarabine with LDAC in a dose-escalation phase I/II study for patients age ≥60 with relapsed/primary refractory AML or high-risk MDS. Aims. Our goals were to estimate the maximum tolerated dose (MTD) and to assess complete remission (CR) rates. Methods. Oral clofarabine was administered daily on days 1-5 together with once daily subcutaneous (SQ) LDAC at 20 mgBID on days 1-10. Adults age ≥60 years with AML or highrisk MDS were eligible if they had primary refractory disease or failed one prior therapy and disease recurred within 1 year of CR date and had ECOG performance status 0-2. Informed consent was obtained from all patients. Results. Twenty-nine patients have been enrolled to date. Median age was 72 (range, 62-82) years. Eight patients (28%) had secondary AML. The median performance status was 1 (range, 0-2) and median time from diagnosis to trial entry was 12 (range, 2-67) days. Cytogenetics (SWOG criteria) were unfavorable in 13 patients (45%), intermediate in 9 (31%), favorable in 1 (3%), and unknown in 6 (21%). The MTD of oral clofarabine when given with LDAC was determined to be 20 mg and the dose-limiting toxicity was cardiac dysfunction. Number of cycles given: 23 patients - 1 cycle, 4 patients - 2 cycles, 1 patient - 3 cycles, 1 patient - 4 cycles. Twenty-six patients were treated as outpatients and 14 patients were hospitalized during the course of treatment including 11 for fever. Seven patients (24%) have achieved a CR. Based on previous MD Anderson data (Blood 1996; 88:756), the expected CR rate with HiDAC, FLAG etc would be 15%. Three patients (10%) achieved CR with incomplete blood count recovery and 1 (3%) a partial remission. Four of the 10 patients who achieved CR or CRi have relapsed at a median of 4 months. The other 6 have been in CR/CRi for a median of 3.5 months (range, 0-13.5 months). Fifteen patients (52%) died after treatment, 10 due toAML. Conclusions. Oral clofarabine at 20 mg daily for 5 days plus LDAC appears to be well tolerated and can generally be delivered in an outpatient setting. The CR rate appears higher than expected. While accrual is on-going, these results warrant further study. FOR TREATMENT OF RELAPSED/REFRACTORY ACUTE LYMPHOBLASTIC LEUKEMIA IN ADULT PATIENTS A Malato1, A Santoro1, R Felice1, D Turri1, S Magrin1, R Di Bella1, R Scimè1, D Salemi1, F Acquaviva1, F Fabbiano1. 1Ospedali Riuniti Villa Sofia-Cervello, U.O. di Ematologia e UTMO, Palermo, Italy Introduction. Relapsed or refractory adult acute lymphoblastic leukemias (ALL) have poor prognosis. The strategy for treating these patients is through reinduction chemotherapy followed by allogeneic stem cell transplantation, provided that the toxicity of the salvage regimen is acceptable. Clofarabine, a next-generation deoxyadenosine analog, has demonstrated significant activity in children and adults with refractory lymphoid and myeloid leukemia in early clinical trials and was granted approval for use in children with acute lymphoblastic leukemia in second or higher relapse. Promising activity of clofarabine in combination with cyclophosphamide, with DNA damage and apoptosis in both AML and ALL blasts, has been reported (Karp JE et al. Blood 2007). Aim. We present a series of ten cases in which clofarabine was combined with cyclophosphamidein adult patients with relapsed or refractory acute lymphoblastic leukemia. Methods. Patients aged 23-59 years with refractory/relapsedALL were treated at the dose of clofarabine 10mg/m2 + cyclophosphamide400g/m2 on days 1-3 and 8-10. We evaluated the overall remission rate (ORR), duration of remission (DOR) and overall survival (OS). Minimal residual disease (MRD) by molecular targeting was considered in all patients. Results. Nine patients received clofarabine 10mg/m2 + cyclophosphamide400mg/m2, both on Days 1-5 and 810;one patient received only one cycle. All patients had relapsed/refractory lymphoblastic leukemia and had received multiple priors tharapies. Eight had pre-B cell ALL, 2 pts had T cell ALL; two pts had received a prior hematopoietic stem cell transplant (HSCT). Four patients achieved a complete remission (CR);two patients went on to receive allogeneic transplants after clofarabine/cyclophosphamide salvage. The median of Overal survival (OS) forall the patients was 103 days, the media was 172,70days. The overall remission rate (ORR) was 44,4%, and we estimated a duration of remission (DOR) as 223,25 days in media (we calculated from the first day of remission). Treatment was complicated by neutropenic fever (n = 4), grade III-IV mucositis (n = 3), prolonged aplasia >30 days (n = 3). One patient died of sepsis before completing the regimen. Conclusion. Combination treatment with clofarabine and cyclophosphamide in adults pts with refractory or relapsed ALL resulted inan ORR of 44%, two ptsproceeded to HSCT. The safety profile is acceptable in this relapsed/refractory population. The response rates and durability of remission observed with this regimen were encouraging given that these patients were highly refractory to prior therapies. However, more studies with this combination in adults are warranted.