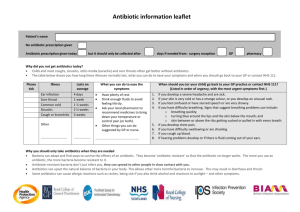
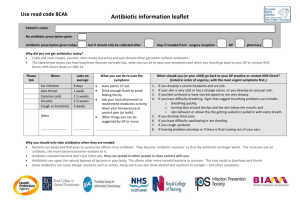
ANTIBIOTICS
advertisement

Biotechnology Annual Review 8, 227-265(2002) Antibiotics V. Běhal Institute of Microbiology, Academy of Sciences of the Czech Republic, Vídeňská 1083, 142 20 Prague, Czech Republic. Abstract. The chapter informes about different types of antibiotics, their structure, biosynthesis and their regulation. Industrial cultivation and isolation of antibiotics is described in the chapter. Search for microorganisms producing antibiotics and preparation of high-producing strains is described. Resistance against antibiotics in producing microorganisms and pathogens is discussed. Keywords: antibiotics, secondary metabolites, overproduction, biological activity, regulation of production, resistance. Introduction Antibiotics as representatives of biologically active substances from microorganisms Antibiotics are defined as microbial products that inhibit growth of other microorganisms. After the antibiotic effect of penicillin had been observed by Fleming, a number of other antibiotics were discovered. The main producers are soil microorganisms as actinomycetes [1], moulds anf fungi (Fig. 1). New antibiotics being searched for the microorganisms were found to produce a broad spectrum of compounds having various effects on living organisms. Some of them have occupied a weighty position as medicines and agricultural drugs and for animal health. One microorganism can produce several compounds with different biological activity (staurosporine) [2] and, on the contrary, one compound can be produced by several microorganisms. Besides to traditional antibiotics, compounds with different biological activities are synthesized by various microorganisms: coccidiostatics used in poultry farming, antiparasitic compounds with a broad spectrum of the activity against nematodes and arthropods, substances with the antitumor activity, immunosuppressors, thrombolytics (staphylokinase), herbicides, pesticides, compounds affecting blood pressure, etc [3]. For medicine are important enzyme inhibitors synthesized by microorganisms [4]. They are used as inhibitors of enzymes produced by resistant strains that decompose the antibiotic during application of antibiotics. These enzyme inhibitors can be also used for inhibition of undesirable enzyme activities in human metabolism that cause some illnesses. Many enzyme inhibitors are protease inhibitors, variously active against pepsin, papain, trypsin, chymotrypsin, catepsin, elastase, renin, etc. Inhibitors of glucosidases, cyclic AMP phosphodiesterase, different carbohydrases, esterases, kinases, phosphatases, etc. have been isolated from microorganisms. The enzyme inhibitors that participate in the biosynthesis of cholesterol and fat are also used in medicine. Several thousands of compounds having different biological activities have so far been listed and new compounds are still isolated from microorganisms. There is a widespread acceptance that microorganisms are an unlimited source of new substances with many potential therapeutic applications. A great number of those compounds, however, are toxic and thus cannot be used for human and veterinary therapy. Role of antibiotics in producing microorganisms Antibiotics are the typical secondary metabolites produced by microorganisms. Secondary metabolites are meant products of microorganisms (also plants) which are not essential for basic metabolic processes such as reproduction and growth [5]. On the other hand, in the case of many secondary compounds, pieces of evidence of their role in the metabolism of the producer have been brought. These compounds often function as the so-called signal molecules, used to control the producer’s metabolism. One of the functions attributed to antibiotics is a suppression of the competing microorganisms in the environment. Thus the antibiotic-producing microorganisms have an advantage in competing for nutrients with the other microorganisms but antibiotic activity is only one from many other biological activities of secondary microbial products. However, the function of antibiotics in the environment can be observed only with difficulty. Use of antibiotics in human, veterinary and plant medicine Antibiotics are very often used in medicine for suppression of pathogenic bacteria, fungi and viral diseases. Their use marked a revolution in medicine, saved millions of lives and helped reduce some, rather frequent diseases such as tuberculosis. An efficient, antiprotozoan antibiotic, however, has not yet been discovered. Antibacterial antibiotics are sometimes used in the case of viral diseases to protect the weakened macroorganism against a subsequent bacterial infection. As mentioned in the introductory part, some antibiotics are also used as cancerostatics or for curing some other illnesses. In a similar way as in human medicine, antibiotics are also employed in veterinary medicine. Besides, antibiotics are added to various feeding mixtures used in poultry and animal farming to keep the animals in good health. If the antibiotics are used, higher farming yields are often reached. However, the administration of antibiotics should be stopped a certain time before the animal is slaughtered and the meat consumed since the residues of antibiotics should not enter the human diet. To avoid the production of strains resistant to the antibiotics used in human medicine, special antibiotics allowed to be employed in veterinary medicine and animal production have been singled out and are no more used in human medicine (chlortetracycline, bacitracin, tylosin, etc.). Side effects of antibiotics. In addition to their positive effects, antibiotics can also have negative effects. Besides various allergies linked with the use of antibiotics, the human organism can sometimes suffer a damage when treated with them. Sometimes toxic compounds can be formed when antibiotics are transformed in the organism. Tetracyclines, that form complexes with calcium, can, for example, inflict damage on the formation of tooth enamel in children, on the condition they are frequently used during the period of teeth growth. A number of newly discovered antibiotics cannot be used for therapy because of their excessive toxicity. Fortunately, the first antibiotic to be used on a massive scale, penicillin, has relatively moderate side effects on the human organism. 2 Biosynthesis In spite of variety of their structures, antibiotics are synthesized from simple building units amino acids, acetate, propionate, sugars, nucleotides which are used in living organisms for the biosynthesis of cellular structures. According to their structure and type of biosynthesis, antibiotics are classified to form several groups. Peptide and peptide-derived antibiotics Peptides Microorganisms produce a number of peptides that have the biological activity. In contrast to biologically active peptides of higher organisms, where they function as hormones, the function of microbial peptides in microorganisms is not known. They are included in the group of secondary metabolites. They differ from the biologically active peptides of higher organisms in having often D-amino acids in their molecules. Besides their antibiotic activity, another interesting feature of the peptide antibiotics is the fact that they are not synthesized on ribosomes, as other peptides, but on enzyme complexes called peptide synthetases [6,7]. Chemical structure. Amino acids linked by the peptide bond form the basal structure of any peptide antibiotic. The peptide chain is often cyclic or branched. In addition to Lamino acids, other compounds can also be present in the molecule, such as D-amino acids, organic acids, pyrimidines and sugar molecules. Numbers of derivatives are known to exist in the case of some peptide antibiotics, that differ in both amino acid substitutions and substituents bound to the amino acids. The linear molecule of gramicidin A and the cyclic molecule of gramicidin S belong to the structurally simplest peptide antibiotics. Bacitracins are an example of cyclic peptides having a side chain (Fig. 2). In the molecule of bleomycins, the sugars Lglucose and 3-O-carbamoyl-D-mannose are found. Peptide antibiotics containing an atom of iron or phosphorus in the molecule have also been isolated. If two molecules of cysteine are present in the peptide antibiotic, they are linked by a sulfide bridge. Another cyclic polypeptide (heptapeptide) is iturin, an antifungal antibiotic, produced by Bacillus subtilis, effective against plant pathogens [8]. A special type of compounds are enniatines. Their molecule consists of three residues of branched amino acids, L-valine, L-leucine and L-isoleucine, and three residues of D2-hydroxyisovaleric acid (D-Hyiv) [9]. The amino acids and D-Hyiv are linked by alternating amide and ester bonds. The amide bonds are finally N-methylated. Molecular conformation is important for the biological activity of peptide antibiotics. This is true mainly for the peptides capable of formation of chelates with metals. The studies showed three-dimensional molecular structures with many hydrogen bonds [10]. In the case of valinomycin (L-Val-D-Hyiv-D-Val-L-Lac)3, that is able to selectively transport K+ and Rb+ ions across natural and artificial membranes, the molecule is symmetrical in three dimensions, if it forms a complex with the metal. If it is not in the form of the complex, it has only a pseudocentral symmetry. 3 Biosynthesis. The biosynthesis of peptide antibiotics takes place on a multienzyme complex [11]. The individual amino acids are activated using ATP to form aminoacyl adenylates. The aminoacyl groups are transferred to the enzyme thiol groups where they are bound as thioesters. The structural arrangement of the thiol groups in the synthetases determines the order of amino acids in the peptide. The formation of peptide bond is mediated by 4-phosphopantetheine, that is an integral part of the multifunctional multienzyme. The way how the order of the amino acids in the molecule is regulated is not known. It is probably determined by the tertiary configuration of the enzyme. This specificity, however, is not very high since the microorganisms mostly produce a mixture of peptides differing only in one or several amino acids in the chain. Enzymes. Gramicidin S synthetase is an enzyme consists of two complementary enzymes having molecular weights of 100 kD and 280 kD. Bacitracin synthetase. The enzyme consists of three subunits [12] (Fig. 3) having molecular weights of 200, 210 and 360 kD [13]. Each subunit contains phosphopantetheine. The enzyme A activates the first five amino acids of bacitracin, the enzyme B activates L-Lys and L-Orn, and the enzyme C activates the other five amino acids. D-amino acids are produced by racemization of their L-forms directly on the enzyme complex. Initiation and elongation start on the subunit A up to the pentapeptide, independently of the presence of the subunits B and C. The pentapeptide is transferred to the subunit B where two other amino acids are added. The heptapeptide is subsequently transferred to the subunit C where the biosynthesis of bacitracin is finished. The cyclization is achieved by binding the asparagine carboxy group to the εamino group of lysine, whereas, to the α-amino group of the same lysine, the isoleucine carboxyl group is bound [7,14]. Mechanism of action. The antibiotic activity of bacitracin results in an efficient inhibition of proteosynthesis and cell wall synthesis but other effects such as an interference with cytoplasmic membrane components and cation-dependent antifungal effects have been observed as well. In the case of gramicidin S, hemolytic effects, inhibition of protein phosphatases and interaction with nucleotides have been observed, in addition to the antibacterial activity. Even though antibiotics normally have several mechanisms of their action, the primary one is thought to be the effect observed at the lowest concentration of all. The peptide antibiotics are efficient mainly against Grampositive bacteria. ß-Lactams. The main representatives of ß-lactams are penicillins and cephalosporins [15,16]. Penicillins have a thiazoline ß-lactam ring in the molecule and differ, one from another, by side chains linked via the amino group. Main types of the penicillin molecule are shown in Fig. 4. Cephalosporins [17] have a basic structure similar to that of penicillins and the derivatives are also formed by a variation of the side chain (Fig. 5). The thiazolidine ß-lactam ring is synthesized using three amino acids: L-α-amino adipic acid, L-cystein and L-valine by α-aminoadipyl-cysteine-valine synthetases [18]. 4 By condensation of these three amino acids, a tripeptide is formed. It is transformed to the molecule of penicillin or cephalosporin through subsequent transformations. The principial works about enzymes of β-lactams biosynthetic pathways were done by Abraham and his collegues [19]. Clavulanic acid also belongs to ß-lactams (6). This acid has a bicyclic ring structure resembling that of penicillin, except that oxygen replaces sulfur in the five-membered ring. Clavulanic acid is an irreversible inhibitor of many ß-lactamases. The discovery of clavulanic acid was a starting point for the development of penicillin analogues, able to inactivate these enzymes. Biological activity. Penicillins are especially active against Gram-positive bacteria but some semisynthetic penicillins, such as ampicillin, that is lipophilic as compared to, for example, benzyl penicillin, are also effective against Gram-negative bacteria. This effect is explained by their easier entering the cells of Gram-negative bacteria that have a high lipid content in the cell wall. ß-lactam antibiotics interfere with the synthesis of bacterial cell wall and thus inhibit bacterial growth. Such a mechanism of action does little harm to the macroorganism to which ß-lactams are applied. Glycopeptides. At present several hundreds compounds belonging to glycopeptides are known, including semisynthetic derivatives. The best known of all is vancomycin (Fig. 7) [20] that is effective against Gram-positive bacteria. This antibiotic is widely used in medicine, especially against ß-lactam resistant microorganisms. Vancomycin is not absorbed from the gastrointestinal tract and is used to treat enterocolitis caused mainly by Clostridium difficile. Vancomycin is produced by several tens of microorganisms, of which Amycolotopsis orientalis is used for commercial production. Glycopeptides are composed of either seven modified or unusual aromatic amino acids or a mix of aromatic and aliphatic amino acids. By the substitution of amino acids in the amino acids core, derivatives of amino glycosides are formed. In vancomycin the aminosugar vancosamine is bound to the amino acids core. The removal of aminosugar reduces the activity of vancomycin two- to fivefold. Glycopeptides lose the majority of their antibiotic activity after splitting of sugar [21]. Polyketide-derived antibiotics A large group of antibiotics includes compounds that are synthesized by polymerization of acetate units and subsequent cyclization of the polyketo chain, that has been formed before or is just being formed, to provide six carbon atoms containing aromatic rings or macrocyclic lactone ring. The terminal group need not be an acetate but also pyruvate, butyrate, ethyl malonate, paraminobenzoic acid, etc. In the early phase, the formation of polyketo chain is similar to that taking place during the biosynthesis of fatty acids, and is catalyzed by the enzyme polyketosynthase [22]. A principal role is played by the Acyl Carrier Protein (ACP) [23]. The ACP prosthetic group in many microorganisms is 4´-phosphopantothenic acid. Its terminal groups and acyls produced by polymerization are bound via the -SH group. The acyls are transferred 5 to the other -SH group, that is a part of the cysteine molecule. Polyketosynthase has not yet been isolated and its properties have been deduced from the analyses of DNA sequences of cloned genes. Polyketosynthases include two distinct groups located either in domains on multifunctional proteins or present on individual, monofunctional proteins. 6-Methyl salicylic acid 6-Methyl salicylic acid (6MS) represents the simplest polyketide, that is formed by condensation and subsequent aromatisation of one acetylCoA molecule and three malonylCoA molecules. This compound was isolated from Penicillium patulum. By other metabolic steps 6MS is transformed to produce a toxin called patulin. The synthesis of 6MS takes place on an enzymatic complex called 6MS synthetase [24,25]. Tetracyclines The chemical structure of typical tetracyclines is shown in Fig. 8 and their biosynthesis in Figs. 9 and 10 [26]. Chlortetracycline and tetracycline are produced by the actinomycete Streptomyces aureofaciens, whereas oxytetracycline and tetracycline by the actinomycete Streptomyces rimosus. As seen in Fig. 9, the tetracycline molecule is synthesized from one molecule of malonic acid semiamide and eight molecules of malonate. In the early steps, the synthesis is similar to the biosynthesis of fatty acids, but the keto groups are not reduced and aromatic rings are formed to yield 6-methyl pretetramide. This compound is the first known intermediate of the tetracycline biosynthesis that is further transformed to yield one of the tetracycline molecules. As to the enzymes transforming the intermediates of chlortetracycline and tetracycline biosynthesis, the last three have been described [27,28]: S-adenosylmethionine:4dedimethylamino-4-aminotetracycline N-methyltransferase methylating the amino group in position 4, anhydrotetracycline oxygenase and NADP:tetracycline 5a(11a)dehydrogenase (tetracycline dehydrogenase). For more extensive coverage of research, articles by Běhal [29] and Běhal and Hunter [30] can by consulted. Tetracyclines act as inhibitors of proteosynthesis. They are considered to be widespectrum antibiotics, that are efficient against both Gram-positive and Gram-negative bacteria. However, having significant side effects on the human macroorganism, they are preferably used only in the case other, less toxic antibiotics are not effective. Anthracyclines Anthracyclines are synthesized in a similar way as other polyketides ([31]. They often have one or several sugar residues in the molecule, most often deoxy-sugars, synthesized from glucose, are present in the anthracycline molecule. As to their biological activity, daunorubicin and doxorubicin (adriamycin) (Fig. 11) are rather important. They are excellent antitumor agents, which are widely used in the treatment of a number of solid tumors and leukemias in human. However, these drugs have dose limiting toxicities such as cardiac damage and bone marrow inhibition. In recent years, a variety of drug delivery systems for anthracyclines have been reported. In most cases, 6 the drugs were linked to high molecular compounds such as dextran [32,33], DNA [34] and others. Macrolides and polyenes Macrolides are usually classified to include: proper macrolides having 12 -, 14- or 16-membered macrocyclic lactone ring to which at least one sugar is bound, and polyenes having 26- to 38-atom lactone ring containing 2 to 7 unsaturated bonds. Besides the sugars bound to the lactone ring, an additional aromatic part is normally present in the polyene molecule. As to the biosynthesis, however, both macrolides and polyenes are synthesized in the same way using identical building units. Macrolides represent a broad group of compounds and new substances have been incessantly added to the list, including hybrid compounds [35]. A number of derivatives of the basic structure can be produced by one microorganism, on the other hand, however, the compounds can also be found in different microorganisms. Macrolides usually possess an antibacterial activity whereas polyenes are mostly fungicides. Erythromycins produced by Saccharopolyspora erythrea (Fig. 12), together with oleandomycin and picromycin, belong to the best known 14-membered lactone ring macrolides. A novel erythromycin was prepared by the recombinant Saccharopolyspora erythrea strain [36,37]. Macrolides with a 16-membered ring are represented by tylosin (Fig. 13) [38], that is produced by Streptomyces fradiae, as well as by leucomycin, spiramycin, etc. The synthesis of lactone ring is similar to that observed in the case of other polyketides. In contrast to aromatics, propionate and butyrate units are more often used in the biosynthesis, instead of acetate ones. The greatest difference, however, consists in the fact that, instead of aromatic rings, a lactone ring is formed. Keto- and methyl groups of the polyketide chain, from which macrolides are formed, are normally transformed more frequently. Nystatin is the best known polyene antibiotic (Fig. 14) [39]. Candicidine is another well known antibiotic belonging to the polyene group. Its molecule includes paminoacetophenone as the terminal group. 4-amino benzoic acid (PABA) was identified as a precursor of the aromatic part of candicidine molecule [40,41]. The sugars found in macrolide and polyene molecules are not encountered in the structures of microbial cells. They include both basic and neutral sugar molecules. Often, L-forms are found. Sofar, at least 15 different sugars have been described to occur in macrolides and polyenes. All of them are 6-deoxy sugars; some of them are Nmethylated, others have the methyl on either the oxygen or carbon atom. As it has been repeatedly proven [42,43], glucose is primarily incorporated into macrolide sugar residues. Also in Streptomyces griseus, glucose, mannose and galactose were incorporated to a greater extent into the mycosamine candicidine, as compared to its aglycon [44]. The transformation of glucose to a corresponding sugar takes place in the form of the nucleoside diphosphate derivatives, which is similar to the situation found in the case of other antibiotics. Avermectins 7 The molecule of avermectins [45] consists of a 16-membered, macrocyclic lactone to which the disaccharide oleandrose is bound (Fig. 15). Avermectins are produced by Streptomyces avermitillis. The macrocyclic ring of avermectins is synthesized, as other polyketides, by producing a chain from acetate, propionate and butyrate building units. Oleandrose (2,6-dideoxy-3-O-methylated hexose) is synthesized from glucose. Avermectins are potent antiparasitic compounds with a broad spectrum against nematode and anthropod parasites. They lack antifungal and antibacterial activities. They bind to a specific, high-affinity site present in nematodes but not in vertebrates. Its dosage for animal and human is extremely low. Ivermectin (22,23-dihydroavermectin B1) is a semisynthetic compound which is used to control internal and external parasites in animals. It is the most potent anthelmintic compound of all. Avermectins are also employed in human medicine and plant protection. Detailed reviews on the uses and biosynthesis of avermectins can be found in recent monographs [46,47]. Chloramphenicol Chloramphenicol (Fig. 16) is produced by Streptomyces venezuelae [48]. However, at present the antibiotic is commercially produced using a fully synthetic process. In contrast to polyketides, the aromatic ring of chloramphenicol molecule is synthesized from glucose via chorismic acid and p-amino benzoic acid in the microbe. Aminoglycosides Streptomycin (Fig. 17) is a well-known representative of aminoglycoside antibiotics. It is synthesized by many streptomycetes to produce a number of derivatives. The molecule of streptomycin consists of three components: streptidine, L-streptose and N-methyl-L-glucosamine. None of these components has been found in the primary metabolism of microorganisms. The biosynthesis of streptomycin was disclosed mainly by Walker [49], who also studied the enzymes participating in the biosynthesis of streptomycin [50]. The importance of streptomycin consists mainly in its efficiency to suppress Mycobacterium tuberculosis. A massive use of streptomycin resulted in effective suppression of tuberculosis, especially in developed countries. Recently, however, the disease caused by M. tuberculosis has been found to increase again due to the occurrence of strains resistant to streptomycin. Antiviral compounds Recently also compouds active against viruses have been discovered. Sattabacins and sattazolins, isolated from Bacillus sp. [51] and fattivirin A1, isolated from Streptomyces microflavus [52] are active against Herpes simplex viruses. Inhibitors of HIV are intensively looked out in microorganisms. Inhibitors of HIV-1 protease were detected in fungus Chrysosporium merdarium P-5626 [53]. A compound which has an inhibitory effect on HIV-1 replication in chronically infected cells as well as actualy infected cells was isolated (after screeninng 10,000 microorganism products) from the culture supernatant of Streptomyces sp. Mer-2487 [54]. A hydroxyl benzaldehyde 8 compound, active against influenza virus in vitro, was isolated from Aspergillus terreus [55]. Rhodopseudomonas capsulata produces a virucide substance which inactivated polio virus, Sindbis virus, some fish viruses, without causing any damage to the host cells [56]. Genetics High production strains The genes coding the enzymes that synthesize antibiotics are mostly located on chromosomes. These genes are called structural genes and the enzymes taking part in the antibiotic synthesis are called the enzymes of secondary metabolism. The structural genes are organized to form one cluster. This situation has been observed in all cases described so far [57,58,59]. The expression of structural genes is controlled in a similar way as in the case of other genes. Next to a cluster of the structural genes, the genes coding for the resistance of the producer to its own antibiotic are located. Those genes are situated either at the beginning or at the end of the cluster, often in both positions. In the case the resistance genes are present in the two positions, different types of resistance are included as a rule. In addition to the structural genes, regulation genes also determine the antibiotic production. They are often located on plasmids. The genetic control of antibiotic biosynthesis is poorly known. The type of control where the antibiotic synthesis is inhibited by the own product can serve as an example. As a result, the product’s cellular concentration is maintained at a physiologically tolerable level and, consequently, the producing microorganism is prevented from being self damaged by high concentrations of the product, that are toxic. Microorganisms that are isolated in nature produce small amounts of antibiotics. They are called “wild type strains”. The term “wild strain”, however, does not reflect the fact that, during selection and subsequent cultivation in the laboratory, a change could occur making it non-identical with the original strain. The term wild type strain thus only refers to the fact that the strain did not undergo an “artificial” genetic change in the laboratory and was duly conserved and maintained. In order that the commercial production of antibiotics could be profitable, higher levels of the antibiotic synthesis are reached via genetic changes. Most often the spores are exposed to UV irradiation, X-rays, gamma-rays, α-particles or chemical mutagens (nitrogen mustards, N-methyl-N´-nitro-N-nitroso guanidine). A combined mutagenesis using various mutagens is currently used. Having germinated, the surviving spores give rise to individual colonies of isolates, whose capability of antibiotic production is then tested. Those of the mutants that exhibit poor growth and sporulation ability are not suitable candidates for further improvement, even though their antibiotic production may exceed that of the original strain. Today’s high production strains, that synthesize as high as 10 000-fold levels of antibiotics, compared to the original strains, must be seen as a result of many year’s, costly efforts. Unfortunately, these high production strains can revert to lose their overproduction. The high production levels have thus to be maintained, at least by a permanent selection of the high production strains during 9 storage, when spontaneous mutagenesis can take place, without involvement of any mutagenic agent. Multiplication of the structural genes is not an important factor increasing the antibiotic production. Mutations resulting in an increased antibiotic synthesis mostly affect the regulatory genes. Hopwood and co-workers [60] transferred the genes for the production of actinorhodine to a low production, wild type strain using a plasmid. Even though the number of copies of the structural gene increased only twofold, the production of the antibiotic rose 30-40 times. The increase of the antibiotic production has to be accompanied by an increase of resistance to the own product. When high production strains are prepared by mutagenesis, a type of mutant that loses some of the structural genes can also be obtained. Such a mutant can exhibit a higher level of an antibiotic intermediate whose transformation stopped due to the absence of the corresponding enzyme. By crossing these mutants, some biosynthetic pathways used to synthesize antibiotics were elucidated, e.g. tetracyclines [61]. Loss of the capability of antibiotic production in the strains where extrachromosomal DNA was removed (e.g. by using acriflavine or ethidium bromide) suggests that the regulatory genes are located on plasmids [62,63,64,47]. Strains that lost the ability to produce other secondary metabolites and nondesirable derivatives of the antibiotic in question are also selected during the process of improvement of high production strains. In this way the metabolic pathways that unnecessarily consume the building units used for the antibiotic synthesis can be eliminated and, also purification of the antibiotic product from other compounds and impurities can thus be facilitated. Genetic manipulation of antibiotic producers The structural genes for a number of antibiotics have been cloned into host microorganisms. Similarly, genes for antibiotic resistance and other regulatory genes have also been cloned. Streptomyces lividans was found to be a suitable acceptor of foreign genetic material, in which a low degree of restriction of this genetic material exists. This microorganism can host various plasmids and phage vectors. However, at the same time, this microorganism was found not to be usable for the synthesis of various antibiotics or of high antibiotic levels. The antibiotic biosynthesis is a very complex process that requires not only the structural genes for enzymes of secondary metabolism but also the genes for regulation of their biosynthesis. Moreover, the overproduction of an antibiotic has to be coordinated with the primary metabolism of the producing microorganism. The cloning of structural genes and genes for resistance to the own antibiotic enables us to work out genetic maps of the antibiotic producers. On the basis of those maps, hybrid clusters combined of two and more clusters of different antibiotics can be created. Consequently, semisynthetic antibiotics can be produced that may possess new biological activities or an antibiotic activity against resistant strains. Polyketide synthase genes of microorganisms producing various polyketides have also been hybridized [22]. As a result, a great similarity of polyketide synthases from 10 various streptomycetes was evidenced and new polyketide antibiotics were synthetized [58]. Search for new antibiotics Isolation from nature At present several thousands of compounds having some biological activity have been obtained from microorganisms isolated from nature. As the probability of finding a new compound that would be usable as a new antibiotic is as little as one in ten thousand, a great number of microorganisms have to be checked. A rough estimation says that about 100 000 microorganisms is screened for the presence of biologically active compounds per year. Well equipped laboratories study about 30 different biological activities. The requirements for new antibiotics result from the occurrence of resistant strains of pathogenic microorganisms, that are no more sensitive to known antibiotics used in the clinical practice. It is mostly big pharmaceutical companies that look for new antibiotics. Their search for new compounds is highly automated. The selection methods used and the methods of detection of the biological activity are normally not published. Preparation of a new antibiotic and its introduction into the clinical practice requires cooperation of scientists from various scientific disciplines. They can be divided into three groups [65]: microbiology (colection of source samples, isolation of diverse microbes, fermentation to enhance the production, taxonomy), pharmacology (target selection, screen design, high-troughput screening, identification of active compounds, efficacy studies, mechanism of action), chemistry (active compounds identification, characterization/replication, isolation/purification, structure elucidation). Producers of antibiotics and other biologically active compounds The majority of the known antibiotics are produced by actinomycetes, fungi and by moulds. With an increasing spectrum of efficiency of microbial metabolites, new, non-traditional sources of such compounds have been looked for. Tropical soils have an enormous biodiversity and they are a rich source of new antibiotics [124]. Various species of microorganisms have been checked including the microorganisms living under extreme conditions (high and low temperatures, etc.), sea living microorganisms [66,67], and higher marine organisms. Marine microorganisms live in a quite different environment from their terrestrial counterparts and would thus be expected to have a different metabolic pathways and to produce compounds, which posses unique structures and activities. The enterprise of screening microbial metabolite for new leads, first exploited by antibiotic researchers and today expanded to virtually all fields of therapeutic interest, has proven successful and will continue as an important avenue to new drug discovery. The original method for determination of the antibiotic efficiency consisted in the application of an extract from the microorganism studied to wells made in a 0.5 cm, agar medium layer in Petri dishes or plates to which the testing microorganism was 11 subsequently inoculated. Most often Staphylococcus aureus, Sarcina lutea, Klebsiella pneumoniae, Salmonella gallinarium, Pseudomonas spp., Bacillus subtilis, and Candida albicans were used. The tests of other biological than antibiotic activities require sophisticated methods. This is true especially when enzyme inhibitors are looked for. Thus, Ogawara et al. [68] chose a tyrosine protein kinase associated with the malignant transformation of the cell caused by retroviruses as the target in a biochemical screen and found genistein, an isoflavone from Pseudomonas, exhibiting a specific inhibitory activity. Production of target enzymes using recombinant DNA methodology has dramatically expanded the number of potential targets that can be feasibly screened. A screen for the inhibitors of HIV reverse transcriptase is an example. The enzyme was produced in Escherichia coli, purified by affinity chromatography, and used to test natural products for the activity [69]. Semisynthetic and synthetic antibiotics After the structures of the antibiotics discovered had been determined and microbial strains resistant to them detected, possible variations of the molecules of known antibiotics were studied. Several methods have been used to accomplish such variations. Biosynthetic antibiotics. The unspecificity of the enzyme systems able to synthesize antibiotics was used, together with the addition of precursors to the growth medium. Thus, the reaction equilibrium was shifted to promote the production of the derivative required. In this way, penicillins with different side chains were prepared. Addition of amino acids to the growth medium can affect the amino acid composition of polypeptide antibiotics. The individual derivatives of penicillin and cephalosporin have slightly different antimicrobial spectra and are capable of suppression of microorganisms resistant to other derivatives. Semisynthetic antibiotics. Replacement of a part of the antibiotic molecule can be accomplished chemically or enzymatically. In this way, semisynthetic penicillins, cephalosporins, tetracyclines, etc. were prepared. The production of semisynthetic penicillins and cephalosporins was facilitated by the fact that 6-amino penicillanic and 7-amino cephalosporanic acids (Fig. 18) could be easily prepared. The side chain is removed by the action of an enzyme or by a chemical hydrolysis and to the amino group in position 6 (penicillins) or 7 (cephalosporins), that was made free in the previous step, another acyl is bound chemically or enzymatically. In such a way, various penicillins and cephalosporins have been prepared to be effective against microorganisms resistant to original compounds. Semisynthetic tetracyclines, pyrolinomethyltetracycline, metamycin and doxycycline, exhibit a greater solubility and somewhat different antimicrobial spectrum, as compared to the original tetracyclines [70]. New derivatives of aminoglycosides have been obtained by chemical and enzymatic modifications. 12 Chemical synthesis of antibiotics As the majority of antibiotics have rather complex structures, their chemical synthesis is mostly more expensive than the production by fermentation. An exception to the rule seems to be chloramphenicol, that is normally prepared using a chemical synthesis. Hybrid antibiotics Using of genetic engineering we can combine structural genes of different antibiotic producers to obtaining new products which are not present in nature [71,72,73]. If these genes are expressed, a hybrid antibiotic is synthesized, that cannot be found in nature. Hopwood et al. [74,60] used this method with the genes of actinorhodin synthesis and obtained related hybrid macrolides, mederhodin A and B, dihydromederhodin A and dihydrogranatirhodin. Niemi et al. [123] prepared new anthracyclines by combination of DNA Streptomyces purpurascens ATCC 15489 and Streptomyces galilaeus ATCC 31615. A next hybrid anthracycline antibiotic was produced by expression of dnrK encoding carminomycin 4-O-methyltransferase in an epelomycin-producing Streptomyces violaceus [75]. Resistance to antibiotics The antibiotic resistance is usually looked at from two angles: first, how the microbial strains arise, that obtain the resistance during the treatment of the macroorganism with the antibiotic, second, the resistance of microorganisms producing antibiotics that build up their resistance against the product of their own which, synthesized at high concentrations, would damage the producer. The ways of how these two types of resistance are achieved are often similar, even though the aims are different. Whereas a resistant microorganism is most often capable of transforming the antibiotic or even degrading it completely, the resistance of producing microorganisms has to ensure that the antibiotic will not be destroyed. Resistance of antibiotic producers The basic metabolic processes of microorganisms producing antibiotics are not inhibited, if the antibiotics are synthesized at low concentrations, observed in strains isolated from nature. By strain improvement, mutants have been able to reach 100 to 1000-fold antibiotic yields, as related to a volume unit of the fermentation medium. Genome changes of the improved strains include a number of deletions and amplifications in the chromosomal DNA. Changes in extrachromosomal DNA were also detected. Low production strains, whose resistance to the own product is low (i.e. higher concentrations of the product inhibit their growth), regulate the antibiotic production, e.g. by inhibiting the enzyme activities that participate in the synthesis of the antibiotic. In high production strains, such a control is lost and the strains have to find a way how to survive in the presence of a high concentration of the antibiotic without decomposing it. 13 As mentioned above, the genes for resistance to the own product are often located at the beginning of the cluster of structural genes. As a result, they are expressed simultaneously with the structural genes. However, the genes of newly gained resistances are mostly located on plasmids. Antibiotics hit active centres of enzymes which makes the enzymes inactive. However, if the enzyme active centre is modified, the antibiotic cannot bind to it and the resistance comes into existence. It is not known whether a decreased ability to bind the antibiotic results from a posttranslational modification of the active centre or if resistant molecules of the enzyme are synthesized de novo. Many antibiotics inhibit protein synthesis, the target site being at the ribosome level. Often, the functions of Tu and G elongation factors are also impaired, together with the synthesis of guanosin penta- and tetraphosphates that is significantly reduced. The antibiotic producers (mostly actinomycetes), as well as the bacteria against which the antibiotic is used, protect themselves by posttranscriptional modification of rRNA. Adenine is methylated to obtain N6-dimethyladenine rRNA in 23S. Such modified ribosomes do not bind the antibiotic. In other cases, adenine is methylated to yield 2-Omethyladenosine [76,77]. However, methylation modified ribosomes can be sensitive to the effect of other antibiotics. The genes coding for methylases, that catalyze methylation of adenine in some streptomycetes, were cloned into Streptomyces lividans and the ribosomes of the mutants prepared were resistant towards the corresponding antibiotics. The most important mechanism of resistance observed in the antibiotic producers seems to be the transport of the antibiotic from the cell to the environment. In the case of high production rates, probably no protection of the active centres could be sufficiently effective. In addition, the antibiotic produced would gradually fill up the interior of the cell. In Streptomyces rimosus, an oxytetracycline producer, genes for the enzymes increasing the antibiotic transport rate precede the structural genes on the chromosome. Genes for the resistance consisting in the protection of ribosomes via the synthesis of an unidentified protein are located at the end of the structural gene cluster [78]. Antibiotic producers also have to solve the problem of a reverse flow of the antibiotic into the cell. Some antibiotics bind to the cell wall, others are complexed in the medium (tetracyclines in the presence of Ca2+ ions). Cytoplasmic membranes of resistant strains are often less sensitive to the effect of antibiotics. This kind of resistance is thought to be connected with the content of phospholipids in the cell. In Bacillus colistinus, a colistin producer, the content of phospholipids in the cell-free extract increased with the sensitivity to the antibiotic. Antibiotic producers can use several types of resistance at one time. Tetracyclines, that strongly inhibit proteosynthesis, interfere with the binding of the ternary complex of amino acyl-tRNA-EFTu-GTP to ribosomes. The genes for resistance were cloned into Streptomyces griseus, sensitive to tetracyclines, using pOA15 as the vector plasmid. After mapping the plasmids in resistant strains using restriction nucleases, two types of plasmids capable of transfer of different types of resistance were found. One type consisted in an increased ability of tetracycline transport to the medium, the other in an increased resistance of ribosomes to the effect of tetracyclines. These ribosomes bore a 14 compound(s), bound to their surface, that could be removed by washing with 1 M NH4Cl solution. The ribosomes lost their resistance after the washing, which was demonstrated with both the ribosomes of Streptomyces griseus and those of the original strain of Streptomyces rimosus. The two types of resistance were both constitutive and inducible [78]. The proteosynthesis inhibiting concentrations of chlortetracycline in Streptomyces aureofaciens are higher in the production phase, as compared to the growth phase [79]. Thus, the resistance can be increased even during the fermentation process. Another way how the antibiotic producers can avoid the effect of their products is by situating the distal enzymes of the antibiotic biosynthetic pathway (synthases) outside the cell, most often in the periplasm. In Streptomyces aureofaciens, a higher proportion of the outside terminal tetracycline synthase was found in production strains under high production conditions in periplasm, as compared to low production conditions [80]. Resistance in pathogenic microorganisms Shortly after antibiotics were introduced into clinical practice on a massive scale, strains of hitherto-sensitive microorganisms started to appear, that required the use of much higher antibiotic concentrations or, even, were completely resistant to these antibiotics. The resistant strains originated from clones that survived the antibiotic treatment, especially if the treatment was terminated before all pathogenic microorganisms were killed or the antibiotic was applied at sublethal doses. There are several ways how microorganisms can gain resistance [81]. In most resistant microorganisms, the main mechanisms of resistance are detoxification or inactivation of the antibiotic, change of the target site, blocking of the transport of the antibiotic out of the cell. Penicillins and cephalosporins are degraded using three ways: a) by the enzyme penicillin amidase that cleaves the amidic bond by which the side chain is bound to the β-lactam ring, b) by the enzyme acetyl esterase that hydrolyzes the acetyl group at C-3 on the dihydrazine ring of cephalosporins, c) by the enzyme β-lactamase that catalyzes hydrolysis of the β-lactam ring of penicillins and cephalosporins. Penicillin amidases are rarely used by microorganisms to build up resistance to βlactam antibiotics. They are often employed for the synthesis of semisynthetic antibiotics. Acetyl esterase is also not important from the point of view of antibiotic resistance. In most cases, β-lactam antibiotics are inactivated by β-lactamase that destroys one of the important sites for their antibiotic activity; the damage is irreversible. However, β-lactamases are not synthesized only by microorganisms that came into contact with penicillins, they have been found in three quarters of all streptomyces strains, their synthesis being constitutive in most of them. One can suppose that the genes for the synthesis of β-lactamases were transferred from microorganisms that possess them in nature to newly arisen resistant strains. Recent studies indicate frequent and promiscuous gene transfer between distantly related bacterial species. A possibility of direct transfer from a streptomycete to a pseudomonad, for example, may seem 15 unlikely. However, it is not necessary to invoke direct exchanges. It is more reasonable to imagine that distant exchanges between distantly related organisms result from a cascade of transfer between related species [82]. Another way of transformation of the antibiotic molecule is N-acetylation of the amino group or O-phosphorylation of the hydroxyl. Bialaphos [83] was found to be inactivated by acetylation. This compound itself is not toxic but, in the cell, phosphinothricine is liberated that inhibits glutamine synthetases, key enzymes of the inorganic nitrogen assimilation in microorganisms and mainly in plants [84]. Regulation of antibiotic production Overproduction of secondary metabolites Microorganisms produce in natural environment only small amount of antibiotics. They have to control the antibiotic synthesis since secondary metabolites at high concentrations are mostly toxic even for their producers. Using high-yielding strains and optimization of fermentation condition we can reach many times higher production. In that case we speak about "overproduction" . Production of antibiotics in factories are at present several thausands higher as production of original strains isolated from nature but this high production is reached only when high-yilding strain is used and special conditons of cultivation are kept. The main factors influence production of antibiotics are discused in next chapters. Growth phases of microbial culture A culture of a microorganism capable of antibiotic production, where the overproduction of the antibiotic is taking place, includes several growth phases representing a number of physiological states. Preparatory phase (lag phase) - the culture is adapting to the new environment, the growth is slow and, evidently, regulatory proteins are being synthesized that, on the basis of the information from the environment, activate the expression of the respective genes during cultivation. Growth phase - the culture grows intensively, usualy a low amount of antibiotic is synthesized. Transition phase - growth rate and proteosynthesis slowed down; the antibiotic production is started. The enzymes of secondary metabolism are intensively synthesized [85,86]. Production phase - growth is practically ceased, dry weight of microorganism is constant, the antibiotic is intensively synthesized. Antibiotic producers mostly belong to filamentous microorganisms (Fig 1B), which means that, in their culture, cells of various age and at different stages of development are present. The microorganisms grow in pellets (Fig. 1A), inside which the cultivation conditions differ from those on the pellet surface (nutrient concentrations, oxygen concentration, etc.). An increase in dry weight does not have to always mean the biomass growth since, in streptomycetes, often a thickening of the cell wall or glycocalyx formation occur that increase the dry weight value without rising the cell number [87]. The individual cells can thus be at different stages of development, i.e. in 16 different physiological states. Therefore, we speak about a physiological state of the whole culture that represents an average of physiological states of the individual cells. Regulation by nutrients In order to reach a high production of an antibiotic, a sufficient biomass yield is necessary, that is accomplished within a short time, if possible. Thus a danger of contamination is diminished and the economic parameters of the fermentation device are kept at its optimum. For this purpose, readily utilizable carbon, nitrogen and phosphorus sources are used. When they are present in the medium, however, an overproduction of the antibiotic does not take place. The culture medium should be designed in such a way that, after the biomass increased sufficiently, at least one of the basic nutrient sources would become depleted and the culture growth would be consequently limited. However, this limitation is not well understood. Regulation by carbon source The inhibition of antibiotic synthesis by glucose was observed shortly after the experiments with penicillin producers started. The antibiotic was found to be synthesized only after glucose was depleted from the medium and lactose started to be metabolized. Similarly, glycerin was observed to inhibit the biosynthesis of cephalosporins [88]. Starch, used as the carbon source, did not inhibit the biosynthesis of penicillin. The concentration of glucose produced from the polysaccharide is that low that the inhibition does not take place. Using these data, fermentation protocols were worked out, in which the level of glucose was kept low so as not to inhibit the antibiotic production. The mechanism of inhibition of the antibiotic synthesis by readily utilizable sugars probably consists in a repression of enzymes of secondary metabolism [89]. In addition to lactose, sucrose, starch and fatty acids are also used as carbon sources in the production phase for the production of antibiotics. Regulation by nitrogen source Readily utilizable nitrogen sources present in the culture medium inhibit the production of antibiotics. Mainly ammonium ions decrease the antibiotic synthesis and, therefore, their concentration in the production media is limited to be exhausted at the end of growth phase. Soy flour, peanut flour and other complex substances are used as nitrogen sources in the production phase of antibiotic fermentations. These nitrogen sources are not easily utilizable and are similar to those used by the microorganisms producing antibiotics in nature. Readily utilizable nitrogen sources repress enzymes of secondary metabolism in Cephalosporium acremonium [90] during the biosynthesis of cephalosporin and in Streptomyces clavuligerus producing cephamycin [91]. Similarly, the inhibition of biosyntheses of leucomycin [92], tylosin [93], and erythromycin [94] are explained by the repression of enzymes of secondary metabolism. Ammonium salts also inhibit the activity of anhydrotetracycline oxygenase isolated from S. aureofaciens [95]. 17 Regulation by phosphate Phosphate is used as main regulator of overproduction of antibiotics in factories. Inorganic phosphate is carefully added in doses to the medium so as to accomplish an optimal ratio between the biomass production and the capability of antibiotic biosynthesis. Bound to organic compounds normally added to the medium (soy flour, etc.), phosphate does not affect the antibiotic production. The antibiotic biosynthesis is started on the condition the concentration of phosphate in the medium decreased below a certain level. Consequently, the producer culture undergoes a shift from the physiological state characteristic for the growth phase to that of the overproduction phase. The influence of inorganic phosphate is explained by repression of the synthesis of enzymes of secondary metabolism [96,97,98]. After the inorganic phosphate was depleted from the medium, a significant decrease of the rate of proteosynthesis was observed during the tetracycline biosynthesis and the synthesis of enzymes of secondary metabolism was commenced [99]. If phosphate was kept above the threshold concentration, the significant decrease of the rate of protein synthesis did not occur and enzymes of secondary metabolism were not synthesized. An addition of phosphate to the medium at the beginning of the production phase, after the phosphorus source was depleted and the enzymes of secondary metabolism synthesis initiated, resulted in a decrease of the enzyme levels in the culture and an acceleration of proteosynthesis. The synthesis of secondary metabolism was resumed after the phosphate was depleted again from the medium. Production of oxytetracycline by Streptomyces rimosus is controlled, at least in part, at the level of transcription from promoters overlapped by tandem repeats similar to those of the DNA-binding sites of the OmpR family [100]. The phosphate was found to be consumed at a higher rate than expected, with respect to the actual rate of protein synthesis, and was probably deposited in the cells in the form of polyphosphates. The capability of absorption of phosphate in the cell seems to be an important function of soil microorganisms. Regulation by metal ions. Metal ions act usually as a part of enzyme active centers. The optimal concentrations of metal ions for cultivation of the antibiotic producing strains have been determined empirically. These ions mostly need not be added to complex media. Some metal ions, present in cultivation media, can substantially decrease production of antibiotics, for instance iron inhibits production of chlortetracycline. Some microorganisms are able to grow in iron-depended medium by secreting iron-regulated chelating compounds as siderophores [101]. Influence of low molecular compounds The antibiotic production can be regulated by different low molecular compounds. The mechanism of their action is not understood. Tryptophan exhibited a stimulatory effect on the production of antibiotics, e.g. mucidin in the basidiomycete Oudemansiella mucida [102] and actinomycin in Streptomyces parvulus [103]. Methionine was found to promote the synthesis of cephalosporin C. Neither tryptophan nor methionine were used as the building units. When enzymes of secondary 18 metabolism were measured, higher levels were detected in the cells of the producing strain. Benzyl thiocyanate increases the production of chlortetracycline and tetracycline in S. aureofaciens. In contrast, it does not influence the production of oxytetracycline in S. rimosus. The effect on the metabolism of S. aureofaciens is multiple [104], including a number of enzymes but the basic influence of benzylthiocyanate at production is the higher expresion of enzymes of secondary metabolism [89]. This is the reason why benzyl thiocyanate is able to raise the antibiotic production only if it is added in the lag phase, growth phase or at the beginning of the production phase. Its effect is more pronounced in low production strains, where the enzymes of secondary metabolism level and the antibiotic production are increased 10 to 20-fold, as compared to high production strains where the increase is only twofold. In the streptomycete antibiotic producers, low-molecular, diffusible compounds have been discovered that regulate the metabolism of the producer, where they are present at very low concentrations, and thus affect both the biochemical and morphological differentiation. The most famous of them is factor A, gamma-butyrolactone (Fig. 19), that was discovered in Streptomyces griseus producing streptomycin [105]. A nonproducing strain started the synthesis of streptomycin after factor A was added to the culture and, in parallel, the formation of aerial mycelium was taking place. Factor A is synthesized by many streptomycetes but the regulatory effect was observed only in Streptomyces griseus, Streptomyces bikiniensis and Streptomyces actuosus. As the result of the addition of factor A to blocked mutants of Streptomyces griseus JA 5142, the synthesis of anthracyclines and leukaemomycin (anthracycline type antibiotic) was resumed. [106]. The resistance to streptomycin linked with an enzymatic phosphorylation of the antibiotic is also induced by factor A [107]. Analogues of factor A have also been found, all of them being gammabutyrolactones. Virginiae butanolides were detected in Streptomyces virginiae [108]. Factor I was isolated from Streptomyces sp. FR1-5 and its effective concentration was 0.6 ng/ml culture. Most of the factor A analogues, however, were not biologically active. Factor B (Fig. 19) was isolated from the yeast Saccharomyces cerevisiae. This substance was capable of eliciting the production of rifamycin in a blocked mutant of Nocardia sp. This substance was effective at a concentration of 10-8 M, when one molecule elicited a synthesis of about 1500 molecules of the antibiotic. The structure of factor B is similar to cAMP but none of the derivatives of known nucleotides exhibited a comparable effect. Chemically prepared derivatives of factor B have also been tested. The effect was observed with those that had a C2 -C12 acyl moiety; octylester was the most effective of them, exhibiting the effect at as low a concentration as 10-10 M [109]. A substitution of guanosine for adenine did not result in a loss of the biological activity of factor B. Factor C was isolated from the fermentation medium of Streptomyces griseus. This compound causes cytodifferentiation of non-differentiating mutants [110]. Factor C is a protein having a molecular weight of about 34 500 D, whose molecule is rich in hydrophobic amino acids. 19 The effect of autoregulators is easily observable, if they elicit morphological changes, such as the formation of aerial mycelium. Carbazomycinal and 6methoxcarbazomycinal, isolated from Streptoverticillium species, were capable of inhibition of the aerial mycelium formation at a concentration of 0.5 to 1 microgram per ml. Autoregulators affecting sporulation were found in Streptomyces venezuelae [111], Streptomyces avermitilis [112]), and Streptomyces viridochromogenes NRRL B1551[113]. From the same strain of Streptomyces viridochromogenes, germicidin was isolated by Petersen and co-workers [114]. The compound had an inhibitory effect on the germination of arthrospores of Streptomyces viridochromogenes at a concentration as low as 40 picograms per ml. Germicidin (6-(2-butyl)-3-ethyl-4-hydroxy-2-pyrone) is the first known autoregulative inhibitor of spore germination in the genus Streptomyces and was isolated from the supernatant of germinated spores, but also from the supernatant of a submerged culture. Mutants of Streptomyces cinnamonensis resistant to high concentrations of butyrate and isobutyrate produce an anti-isobutyrate factor, that is excreted into the culture medium [115]. On plates, anti-isobutyrate factor efficiently counteracted toxic concentrations of isobutyrate, acetate, propionate, butyrate, 2-methylbutyrate, valerate, and isovalerate in Streptomyces cinnamonensis and other Streptomyces species. General control mechanisms have been looked for that operate in the antibiotic biosynthesis. The energetic state of the cell is thought to be such a general control mechanism. The intracellular ATP level reflects the content of free energy in the cell. In some cases, the start of the antibiotic synthesis is linked with a decrease of the intracellular ATP level. Such a relationship was observed in Streptomyces aureofaciens and Streptomyces fradiae during the production of tetracycline [116,117] and tylosin [118], respectively. Even though the regulatory role of ATP cannot be strictly excluded, the results seem to support a hypothesis that a higher ATP level is accompanying the active primary metabolism. A slow down of growth and of the whole primary metabolism would logically be accompanied by a decrease of the ATP level. As in the case of ATP, the role of cAMP in the metabolism of antibiotic producers was also studied, especially in connection with the glucose regulation. Hitherto, no indication has been obtained suggesting a significant role of cAMP in the regulation of antibiotic production [119]. Reception of signals from environment The way of reception of signals from the environment, so that they would be available to the genetic material of the cell to result in the initiation of the antibiotic synthesis, is known quite well. It does not significantly differ from the trasduction of signals for other metabolic processes. Catabolite repression signals or those signalling the depletion of nitrogen or phosphate or the initiation of sporulation are transducted via two-component, signal proteins [120]. In spite of some structural varieties, these proteins are characterized by general mechanistic features and conserved amino acid sequences. The two-component system consists of a cytoplasmic membrane-linked, sensor-transmitter protein and a response-regulator protein, located in the cytoplasm. The sensor-transmitter is composed of a sensor domain located near its N-end; the N- 20 end is found outside the cytoplasm. A specific effector is capable of binding directly to this N-end. The transmitter domain is located in the cytoplasm to be linked to the sensor domain via a hydrophobic, amino acid sequence stretching across the membrane. The sensor-transmitter proteins are normal histidine-protein kinases, capable of autophosphorylation at its C-end on receiving a proper signal. The phosphorylated protein becomes a donor in reactions transferring phosphorus. The acceptor is the cytoplasmic, response-regulator protein. Two-component signal proteins thus transfer the information concerning the conditions that can affect the cell action. Response-regulator characteristic Response-regulator proteins usually contain 200-500 amino acids. The phosphorylation of the aspartic acid carboxyl near the N-end of the response regulator can represent an energy rich bond whose energetic level is believed to decrease through a conformation change of the protein. The above mentioned type of regulator contains a DNA binding protein located near its C-end. If the conformation of this protein is changed, it becomes capable of an interaction with a specific DNA sequence. Transcription initiation of structural genes Regulatory proteins, having been bound to specific DNA sequences and having interacted with RNA polymerase, start the transcription. Regulatory proteins that activate the transcription of structural genes are probably synthesized already during the lag phase. Their binding to DNA and a subsequent biosynthesis of the antibiotic depend mainly on the composition of the growth medium. Provided inorganic phosphate is present in the medium, the activator becomes phosphorylated and thus incapable of binding to DNA. In contrast, for example, the activator of the synthesis of glutamine synthetase, a key enzyme of the assimilation of ammonium salts from the medium and, consequently, of utmost importance for proteosynthesis, is able to bind to DNA only in a phosphorylated form. One can hypothesize that a depletion of inorganic phosphate from the medium does not stop proteosynthesis as a result of a lack of phosphate in the cell for the biosynthesis of cellular structures, as the phosphate limitation is normally explained, but rather the presence or absence of phosphate in the medium causes respective activation or repression of the activators of the enzyme syntheses in primary or secondary metabolisms. This idea is also supported by the fact that enzymes of secondary metabolism were synthesized and the antibiotics produced immediately after the phosphate, that had been added at the beginning of the production phase, was depleted from the medium and deposited in the cell [89]. Technology of antibiotic production Some antibiotics are commercially produced on a ton scale. The fermentation process during which microorganisms produce antibiotics is carried out in fermentors having a volume of several tens of cubic meters. As in any fermentation process, a conserved strain is used, that is first propagated in the laboratory and then in a plant fermentor. The 21 cells are then used to inoculate production fermentors. The inoculum is most often put into a 10 to 20-fold volume of the fresh medium. Isolation of a producing microorganism from one cell The spores are transferred from an agar slope into a volume of 10 ml of sterile H2O and, after homogenization, the suspension is diluted to contain 30-50 spores (Fig. 1D) in 1 ml. A volume of 0.25 ml of this suspension is transferred on the surface of a suitable agar medium on a Petri dish and spread with a sterile glass stick. Colonies, each of which originates from one cell, grow on the agar. The individual colonies are reinoculated to agar slopes and their antibiotic production is tested. Conservation of microorganisms If cultures are conserved for a long time on agar slopes, being repeatedly transferred from one slope to another, they can degenerate and lose valuable technological properties. Two types of conservation are recommended for long term storage of strains: lyophilization (microbial cells or spores are conserved by quick removal of water by sublimation at a low temperature) or conservation by keeping cultures at a very low temperature (-70oC) in liquid nitrogen. In both cases cultures keep their properties for at least 10 years. Laboratory cultivation Cultivation in the laboratory, irrespective of the fact whether the microorganism will finally be used for inoculation of a production fermentor or in laboratory experiments, is carried out in test tubes or in 200-1000-ml bottles and flasks. The volume of the culture medium mostly represents about one tenth of the total volume of the flask. The flasks are sealed with stoppers allowing diffusion of the air into the flasks to ensure aerobic conditions for growth. At the same time, the stoppers prevent microorganisms from the environment to penetrate into the flasks (cotton-wool stoppers, etc.). Producers of antibiotics require a proper aeration, that is important for both the growth and production of the antibiotic. Therefore, the flask contents is well mixed by agitation on rotary or reciprocal shakers placed in thermostated rooms or boxes. Strictly sterile conditions have to be ensured for the cultivation of antibiotic producers since, in the case of contamination, the producing culture can be suppressed by more rapidly growing microorganisms. Cultivation in fermentors Microbial producers of antibiotics are cultivated in fermentors of various size. The lower limit of size of laboratory fermentors is about 1 litre. Owing to the use of complex media, foam is often formed during cultivation and, therefore, the fermentors are filled with the medium up to one half or two thirds of their maximal capacity. When the process of antibiotic production is scaled up from the laboratory conditions to those of true production, basic parameters can be established using several-litre, laboratory fermentors. However, they should be verified in pilot plant fermentors having a size of several cubic meters. The basic equipment of both laboratory and pilot plant fermentors 22 is practically the same. They are made of inert materials such as glass and stainless steel, or their walls are at least lined with an inert material. The fermentors are equipped with a device keeping the cultivation temperature constant (mainly cooling device is important in large fermentors) and with an efficient aeration system, since antibiotic producers require a sufficient oxygen supply for both the growth and synthesis of the antibiotic. The aeration systems based on intensive stirring are not suitable for cultivation of antibiotic producers since a majority of them are filamentous microorganisms that can suffer damage when intensively stirred. The air flowing into the fermentor has to be sterile. It is sterilized by filtration; most often glass wool or mineral wool filters are used. The fermentor is also usually equipped with a device ensuring automatic dosage of antifoaming agents. Antifoaming agents, which increase interfacial tension, are used for breaking the foam. Their amount should be minimal because they decrease solubility of oxygen in the medium. Mixtures of various fatty acids (mainly oleic acid) or some synthetic oils are used as antifoaming agents. An automatic foam breaker consists of a probe that ensures the contact with the foam and, at the moment the contact is realized, an impulse is given by the probe to the injector to add a small amount of the antifoaming agent to the fermentor. In order that the pH of the culture could be adjusted during cultivation, fermentors are equipped with a device ensuring automatic dosage of acids and bases. Most antibiotics are produced in a fed batch system, i.e. a certain amount of the culture medium is inoculated with the producing microorganism and, after a time interval, another dose of nutrients is added to the fermentor. Thus a prolonged cultivation can be accomplished that enables us to increase the yield of the antibiotic. The inflow of nutrients makes possible keep their optimal levels. In cultivations whose course is well known, the nutrient inflow is programmed in advance. Solid-state fermentation Solid-state, or substrate, fermentation is characterized by a fermentation process on a solid support, which has a low moistre content and occurs in a non-septic and natural state [120]. The use of solid-state technology for the production of antibiotics has some advantages. Due to the lack of free water, smaller fermentors are required and the mycelial microorganisms, used predominantely for antibiotic production are well suited to grow on a solid support. On the contrary, a liquid fermentation process allows greater control and monitoring of parameters, such pH, heat, nutrient condition etc [121]. Isolation, separation and purification of antibiotics Isolation of an antibiotic from the fermentation medium depends on the fact whether the antibiotic is secreted into the medium or remains in the biomass, inside the cell or bound to the cell wall. If the antibiotic is bound to the biomass or, in contrast, present in the broth supernatant, the two phases are separated by filtration or centrifugation and extracted separately. If the antibiotic is present in both phases the whole broth is used for extraction. Another isolation step usually includes an extraction with solvents of different polarities, followed by evaporation of the extracts to dryness. 23 If the antibiotic is extractable by nonpolar solvents, the extraction is preceded by dehydration, most often using methanol or acetone. By the extraction with nonpolar solvents, a most part of water soluble compounds present in the medium is eliminated. The crude isolate obtained is used as a material for further separation processes. The antibiotic producers often synthesize a number of compounds or derivatives of the desired compound that have to be separated from the antibiotic produced. The separation is carried out using standard operations such as an extraction into another solvent, chromatography techniques and, in the end, precipitation or crystallization. The purity of the antibiotic compound is checked by thin-layer chromatography, gass chromatography or HPLC. In the case of new compounds, their structure is determined using spectroscopical methods such as nuclear magnetic resonance, mass spectroscopy, and IR, UV or visible-light spectroscopy References 1. Běhal V. Bioactive products from Streptomyces. Adv Appl Microbiol 2000;47:113156. 2. Omura S, Sasaki Y, Iwari Y and Takeshima H. Staurosporine, a potentially important gift from a microorganism. J Antibiot 1995;48:535-548. 3. Běhal V. Nontraditional microbial bioactive metabolites. Folia Microbiol 2001;46: (6) 000-000, (in press). 4. Umezawa K, Aoyagi T, Suda T, Hamada M and Takeuchi T. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J Antibiot 1976;30:170-173. 5. Bennett JW and Bentley R. What is a name?-Microbial secondary metabolites. Adv Appl Microbiol 1989;35:1-28. 6. Lipman F. Attempts to map a prcoess evolution of peptide biosynthesis. Science 1971;173:875-884. 7. Laland SG and Zimmer T-L. Bioactive peptides produced by microorganisms. Essay Biochem 1973;9:31-57. 8. Ohno A, Ano T and Shoda M. Production of the antifungal peptide antibiotic, iturin, by Bacillus subtilis NB 22 using wheat bran as a substrate. Biotechnol Lett 1992;14:817-822. 9. Billich A and Zocher R. Enzymatic synthesie of cyclosporine A. J Biol Chem 1987;262:17258-17259. 10. Iitaka Y. Molecular conformations of bioactive peptides in crystals, In: Umezava H, Takita T and Shiba T (eds) Bioactive Peptides by Microorganisms, Kadansha, Tokyo, 1978;153-182. 11. Kleinkauf H von Doehren H In: Kleinkauf H., von Doehren H, Dornauer H and Nasemann G (eds) Regulation of Secondary Metabolite Formation, VCH Verlagsgesselshaft, Weinheim, 1986:173-207. 24 12. Roland I, Froyshov O and Laland G. A rapid method for the preparation of three enzymes of bacitracin synthetase essentialy free from other proteins. FEBS Lett 1977;84:22-24. 13. Ishihara HM, Hara N and Iwabuchi T. Molecular cloning and expression in Escherichia coli of Bacillus licheniformis bacitracin synthetase gene 2 gene. J Bacteriol 1989;171: 1705-1711. 14. von Doehren H. In: Vining LC and Stuttard C (eds) Genetics and Biochemistry of Antibiotic Production, Butterworth-Heinemann, Boston, 1995;129-171. 15. Martin JF and Liras P. Beta-lactams. Adv Biochem Eng 1989;39:153-187. 16. Jensen SE and Demain AL. In: Vining LC and Stuttard C (eds) Genetics and Biochemistry of Antibiotics Production, Butterworth-Heinemann, Boston, 1995;239268. 17. Newton GGF and Abraham EP. Isolation of cephalosporin C, penicillin-like antibiotic containing D-α-aminoadipic acid. Biochem J 1956;62:651-658. 18. Martin JF. Α-aminoadipyl-cysteinyl-valine santhetases in β-lactam producing organisms. J Antibiot 2000;53:1008-1021. 19. Abraham EP. In: Kleinkauf H, von Doehren H, Dornauer H and Nesemann G (eds) Regulation of secondary metabolite formation. VCH Verlagegesselshaft, Weinheim, 1986;115-132. 20. Parenti F and Cavalleri B. Proposal to name the vancomycin-ristoceti like glycopeptides as dalbaheptides. J Antibiot 1989;42:1882-1883. 21. Williams DH, Stone MJ, Mortishire-Smith RJ and Hauck PR. Molecular recognition by secondary metabolites. Biochem Pharmacol 1990;40:27-34. 22. Bentley R and Bennett JW. Constructinc polyketides: From Collie to combinatorial biosynthesis. Ann Rev Microbiol 1999;53:411-446. 23. Hopwood DA and Sherman DH. Molecular genetic of polyketides and its comparison to fatty acid biosynthesis. Ann Rev Genet 1990;14:37-66. 24. Dimroth P, Walter H and Lynen F. Biosynthesis von 6-methylsalicylsaure. Eur J Biochem 1970;13:98-110. 25. Dimroth P, Ringelmann E and Lynen F. 6-methylsalicylic acid from Penicillium patulum. Eur J Biochem 1976;68: 591-596. 26. McCormick JRD. In: Vaněk Z and Hošťálek Z (eds) Biosynthesis of Antibiotic Substances. Academic Press, Praha, 1965;73-79. 27. Miller PA, Saturnelli A, Martin JH, Mitcher LA and Bohonos N. A new family of tetracycline precursors: N-demethylanhydrotetracyclines. Biochem Biophys Res Commun 1964;16:285-291. 28. Miller PA, Hash JH, Lincks M and Bohonos N. Biosynthesis of 5hydroxytetracycline. Biochem Biophys Res Commun 1965;18:325-331. 25 29. Běhal V. Tetracycline fermentation at its regulation. CRC Crittical Reviews in Biotechnology 1987;5:275-318. 30. Běhal V and Hunter IS. In: Vining LC and Stuttard C (eds) Genetics and Biochemistry of Antibiotics Production. Butterworth-Heinemann, Boston, 1995;359384. 31. Hutchinson CR. In: Vining LC and Stuttard C (eds) Genetics and Biochemistry of Antibiotics Production, Butterworth-Heinemann, Boston, 1995;331-357. 32. Levi-Schaff F, Bernstein A, Meshore A and Arnon R. Reduced toxicity of daunorubicin by conjugation to dextran. Cancer Treat Terp 1982;66:107-114. 33. Tanaka H, Kominato K, Yamamoto R, Yoshika T, Nishida H, Tone H and Okamoto R. Synthesis od doxorubicin-cyclodextrin conjugates. J Antibiot 1994;47:1025-1029. 34. Campeneere DD, Baourain R, Huybrechts M and Trouet A. Comparative study in mice of the toxicity, pharmacology, and therapeutic activity of daunorubicin-DNA and doxorubicin-DNA complex. Chem Pharm Bull 1979;37:1639-1641. 35. Nakagawa A and Omura S. Biosynthesis of bioactive microbial metabolites and its aplication to the structural studies and production of hybrid compounds. J Antibiot 1996;49:717-741. 36. Pacey MS, Dirlam JP, Geldart RW, Leadlay PF, McArthur HAI, McCormick EL, Monday RA, O´Connell TN, Staunton J and Winchester TJ. Novel erythromycins from recombinant Saccharopolyspora erythrea Strain NRRL 2338 pIG/1 I. Fermentation, isolation and biological activity. J Antibiot 1998;51:1029-1034. 37. Parson IC, Everett JR, Pocey MS, Ruddock JC, Swanson AG and Thomson CM. Structural elucidation of a novel erythromycin, 13-cyclopentenyl-13-desethyerythromycin B, from recombinant Saccharopolyspora erythrea Strain, NRRL 2338 pIG/1. J Antibiot 1999;52:190-192. 38. Omura S, Nakagawa A, Takeshima H, Miyazava J and Kitao C. A 13C nuclear magnetic study of the biosynthesis the 16-membered macrolide antibiotic tylosin. Tetrahedron Lett 1975; 4503-4506. 39. Manwaring DG, Rickards RW, Guadiano G and Nicolella V. J Antibiot 1969;22:545-550 40. Liu CM, McDanie LE and Schaffner CP. Studies on candicidin biosynthesis. J Antibiot 1972;25:116-212. 41. Martin JF. Biosynthesis of polyene macrolide antibiotics. Ann Rev Microbiol 1877;31:13-38. 42. Corcoran JW and Chick M. In: Snell P (ed) Biosynthesis of Antibiotics, Academic, New York, 1966;159-201. 43. Salah-Bey K, Doumith M, Michel JM, Haydock S, Cortes J, Leadlay PF and Raynal MC. Targeted gene inactivation for the elucidation of deoxysugar biosynthesis in the erythromycin producer Saccharomyces erythrea. Mol Gen Genet 1998;257:542-553. 26 44. Martin JF and Gil JA. Biosynthesis and attachment of amminosugars to polyene macrolide antibiotics. J Antibiot 1979;32:5122-5128. 45. Burg RW, Miller BM, Baker EE, Birnbaum J, Currier SA, Hartman R, Yu-Lin K, Monaglan RL, Olson G, Putter I, Tunac JB, Wellick H, Stampley EO, Oiwa R and Omura S. Avermectins, new family of potent anthelminthic agens: Production organism and fermentation. Antimicrob Agents Chemother 1979;15:361-367. 46. MacNeil DJ. In: Vining LC (ed) Genetics and Biochemistry of Production, Stuttard, Butterworth-Heinemann, Boston, 1995;421-442. Antibiotic 47. Ikeda H and Omura S. Control if avermectin biosynthesis in Streptomyces avermectilis for the selective production of useful component. J Antibiot 1995;48:549562. 48. Vining LC and Westlake DWS. In: Vandamme EJ (ed) Biotechnology of Industrial Antibiotics, Marcel Dekker, Inc, New York, 1964;387-411. 49.Walker MS and Walker JB. Streptomycin biosynthesis. J Biol Chem 1971;246:70347040.51 50. Walker JB. In: Hash JH (ed) ATP: Streptomycin 6-phosphotransferase. Methods in Enzymology, 1975;43:428-470. 51. Lampis G, Deidda D, Maulla C, Madeddou MA, Pompei R, Monache FD and Satta G. Sattabacins and sattazolines: New biological active compounds with antiviral properties extracted trom Bacillus sp. J Antibiot 1995;48,516-519. 52. Uyeda M, Yokomizo K, Miyamoto Y and Habid E-SE. Fattyviracin A1, a novel antihereptic agent produced by Streptomyces microflavus Strain No. 2446. I. Taxonomy, fermentation, isolation, phasico-chemical properties and structure elucidation. J Antibiot 1998;51:823-828. 53. Fredenhagen A, Petersen F, Tintelnot-Blomley M, Roesel J, Mett H and Hug P. Semicochliodinol A and B: Inhibitors of HIV-1 protease and EDF-R protein tyrosine kinase related to asterriquinones produced by the fungus Chrysosporium merdarium. J Antibiot 1997;50:395-401. 54. Takeuchi H, Asai A, Tanabe K, Kozaki T, Fujita M, Sakai T, Okuda A, Naruse A and Yamamoto S. EM2487, a novel anti-HIV-1 antibiotic, produced by Streptomyces sp. Mer-2487: Taxonomy, fermentation, biological properties, isolation and structure elucidation. J Antibiot 1999;52;971-982. 55. Nishihara Y, Tsujii E, Yamagishi Y, Sakamoto K, Tsurumi Y, Turukawa S., Ohtsu R, Kino T, Hino M, Yamashita M and Hashimoto S. FR198248, a new anti-influenza agent isolated from Aspergillus terreus No. 13830 I. Taxonomy, fermentation, isolation, physico-chmical properties and biological activities. J Antibiot 2001;54:136-143. 56. Hirotani H, Ohigashi H, Kobayashi K and Takahashi E. Inaktivation of T5 phage by cis-vaccinic acid, an antivirus substance from Rhodopseudomonas capsulata, and by unsaturated fatty acids and related alcohols. FEMS Microbiol Lett 1991;77:13-18. 27 57. Binnie B, Warren M and Butler MJ. Cloning and heterologous expression in Streptomyces lividans of Streptomyces rimosus genes involved in oxytetracycline biosynthesis. J Bacteriol 1989;171:887-895. 58. Malpartida F and Hopwood D A. Molecular cloning of the whole biosynthetic patway of a Streptomyces antibiotic and its expression in a herogenous host. Nature 1984;309:462-464. 59. Lotvin JA, Ryan MJ and Strahty N. European Patent Application 1992;91110631,8. 60. McCormick JRD, Hirsch U, Sjolander NO and Doerschuk AP. Cosynthesis of tetracyclines by pairs of Streptomyces aureofaciens mutants. J Am Chem Soc 1960;82:5006-5009. 61. Hotta K, Okami Y, Umezawa H, Huang M and Gipson F. Elimination of the ability of kanamycin-producing strain to biosynthesis deoxystreptamine moiety by acriflavine. J Antibiot 1977;30:1146-1149. 62. Okanishi M. Function of plasmids in aureothricin production. Trend in Antibiot Res 1985;32-41 63. Akagava H , Okanishi M and Umezava H. Genetics and biochemical studies of chloramphenicol nonproducing mutants of Streptomyces venezuelae carrying plasmid. J Antibiot 1979;32:610-620. 64. Yarbrough GG, Taylor DP, Rowlands RT, Crawford MS and Lasure LL. Screening microbial metabolites for new drugs-teoretical and practical issues. J Antibiot 1993;46:535-544. 65. Shiozava H, Kagasaki T, Torikata A, Tanaka A, Fujimota K, Hata T, Furukawa Y and Takahashi S. Thiomarinols B and C, new antimicrobial antibiotics produced by a marine bacterium. 1995;48:907-909. 66. Nagao T, Adachi K, Sakai M, Nishijima M and Sano H. Novel macrolactins as antibiotic lactones from a marine bacterium. J Antibiot 2001;54:333-339. 67. Ogawara H, Akiyama T, Ishida J, Watanabe S and Suzuki K. A specific inhibitor for tyrosine protein kinase from Pseudomonas. J Antibiot 1986;39:606-608. 68. Take Y, Inouye Y, Nakamura S, Allaudeen HS and Kubo A. Comparative studies of the inhibitory properties of antibiotics on human immunodeficiency virus reverse transcriptase and cellular DNA polymerases. J Antibiot 1989;42:107-115. 70. Sum FJ, Sum FW and Projan SJ. Recent development in tetracycline antibiotics. Curr Pharm Res 1998;4:119-132. 71. Hutchinson CR. The inpact of genetic engineering on the commercial production of antibiotics by Streptomyces and related bacteria. Appl Biochem Biophys 1987;16:169190. 72. Hutchinson CR. Prospects for the discovery of new (hybrid) antibiotics by genetic engineering of antibiotic-producing bacteria. Medicinal Res Rev 8;558-567. 28 73. Hopwood D. Genetic enginering of Streptomyces to create hybrid antibiotics. CurrOpin Biotechnol 1993;4:531-537. 74. Hopwood DA, Malpartida F, Kieser HM, Ikeda H and Duncan J. Production of "hybrid" antibiotics by genetic engineering. Nature 1985;314:624-644. 75. Miyamoto Y, Ohta S, Johdo O, Nagamatsu Y and Yoshimoto A. Production of a new hybrid anthracycline 4-O-methylepelmycin by heterologous expression of dnrK in epelmycin-producing Streptomyces violaceus. J Antibiot 2000;53:828-836. 76. Cundliffe E and Thompson J. Ribosome methylation and resistance to thiostrepton. Nature 1979;278:859-861. 77. Mikulík K, Jiráňová A, Janda I and Weiser J. Suscepcibility of ribosome of the tetracycline producing strain of Streptomyces aureofaciens to tetracycline. FEBS Lett 1983;152:125-130. 78. Ohnuki T, Katoh T, Imanaka T and Aiba S. Molecular cloning of tetracycline resistance genes from Streptomyces rimosus in Streptomyces griseus and characterization of the cloned genes. J Bacteriol 1985;161:1010-1016. 79. Běhal V, Vaněk Z, Hošťálek Z and Ramadan A. Synthesis and degradation of proteins and DNA in Streptomyces aureofaciens. Folia Microbiol 1979a;24:211-215. 80. Erban V, Běhal V, Trilisenko L, Neužil J and Hošťálek Z. Tetracycline dehydrogenase: Specroscopic assay properties and localization in strain Streptomyces aureofaciens. J Appl Biochem 1985;7:341-346. 81. Ogawara H. Antibiotic rezistance in pothogenic and producing bacteria, with special reference to β-lactam antibiotics. Microbiol Rev 1981;45:591-619. 82. Davis J. Another look at antibiotic rezistance. J Gen Microbiol 1992;138:15531559. 83. Bayer H, Gungel KH, Hagele K, Hagenmayer H, Jessipow S, Koenig WA and Zaehner H. Stoffwechselproducte von Microorganismen. Helv Chim Acta 1972;55:224-239. 84. Donn G, Tischer E, Smith JA and Goodman HM. Bialaphos, as well as phosphinotricine, inhibits the activity of glutamine synthetase herbicide-rezistant alfalfa cells: An example of gene amplification in plants. J Mol Appl Genet 1984;2:621-635. 85. Běhal V. Enzymes of secondary metabolism in microorganisms. Trends Biochem Sci 1986a;11: 88-91. 86. Běhal V. In: Kleinkauf H, von Doehren H, Dornauer H and Nasemann G (eds) Regulation of Secondary Metabolite Formation, VCH Verlagsgesselshaft, Weinheim, 1996b;269-281. 87. Voříšek J, Čurdová E, Jechová V, Lenc B and Hošťálek Z. Electron-cytochemical demonstration of polyphosphates and the appropriete phosphates in the glycocalyx of Streptomyces aureofaciens. Cuerrent Microbiology 1983;8:31-36. 29 88. Demain AL. In: Demain AL and Solomon NA (eds) Handbook of Experimental Pharmacology, Springer Verlag, 1983;67:189-228. 89. Erban V, Novotná J, Běhal V, and Hošťálek Z. Growth rate, sugar consumption and the expression of anhydrotetracycline oxygenase in Streptomyces aureofaciens. Folia Microbiol 1983;28:262-267. 90. Shen YC, Hein J, Solomon NA Wolfe S and Demain AL. Represion of ß-lactam production in Cephalosporium acremonium by nitrogen sources. J Antibiot 1984;37:503-512. 91. Demain A and Braňa AF. In: Kleinkauf H, von Doehren H, Dornauer H and Nasemann G (eds) Regulation of Secondary Metabolite Formation, VCH Verlagsgesselshaft, Weinheim, 1986;77-88. 92. Omura S, Tanaka Y, Takahashi Y and Iwai Y. Stimulation of leucomycin production by magnesium phosphate and its relevance to nitrogen catabolite regulation. Antimicrob Agents Chemother 1980a;18:691-695. 93. Omura S, Tanaka Y, Takahashi Y and Iwai Y. Stimulation of the production of antibiotics by magnesium phosphate and related insoluble materials. J Antibiot 1980b; 33:1568-1569. 94. Flores E and Sanches S. Nitrogen regulation of erythromycin formation in Streptomyces erythreus. FEMS Microbiol Lett 1985;26:191-194. 95. Běhal V, Neužil J and Hošťálek Z. Effect of tetracycline derivations and some cationts on the activity of anhydrotetracycline oxygenase. Biotechnol Lett 1983;5:537542. 96. Běhal V. Hošťálek Z and Vaněk Z. Anhydrotetracycline oxygenase activity and biosynthesis of tetracyclines in Streptomyces aureofaciens. Biotechnol Lett 1979b;1: 177-182. 97. Madry N and Pape H. In: Schall KP and Pulverer G (eds) Actinomycetes. Zbl Bact Suppl, G. Fischer, Stutgart, New York, 1981; 441-445. 98. Martin JF, Alegre MT, Gil JA and Naharro G. In: Vezina C and Singh K. (eds) Advances in Biotechnology: Fermentation Products, Pergamon press, Toronto, 1981;3:129-134. 99. Běhal V. In: Krumphanzl V, Sikyta B, Vaněk Z and Tempest DW (eds) Overproduction of Micobial Products. Academic Press, London, 1982;301-309. 100. McDowall KJ, Thamchaipenet A and Hunter IS. Phosphate control of oxytetracycline production by Streptomyces rimosus is at the level of transcription from promoters overlapped by tandem repeats similar to those of the DNA-binding sites of the OmpR family. J Bacteriol 1999;181:3023-3032. 101. Nudel C, Gonzales R, Castaňeda A, Mahler G and Actis LA. Influence of iron on growth, production od siderophore compounds, membrane proteins, and lipase activity in Acinetobacter calcoaceticus BD 413. Microbiol Res 2001;155:263-269. 30 102. Nerud F, Zouchová Z and Musílek V. Effect of tryptophan on ezymes of aromatic acids metabolism in Oudemansiella mucida. Folia Microbiol 1984;29:389-402. 103. Troast T, Hitchcock MJM and Katz E. Distinct kinureninase and hydroxykinunerinase enzymes in an actinomycin-producing strain of Streptomycec paravulus. Biochem Biophys Acta 1980;612:97-106. 104. Novotná J, Li Xin-Ming, Novotná JJ, Vohradský J and Weiser J. Protein profiles of Streptomyces aureofaciens producing tetracyclines. Reappraisal of the effect of benzyl thiocyanate. Current Microbiol 1995;31:84-91. 105. Khokhlov AS. In: Krumphanzl V, Sikyta B, Vaněk Z and Tempest DW (eds) Overproduction of Micobial Products, Academic Press, London, 1982;97-109. 106. Graefe U, Schade W, Eritt I and Fleck WF. A new inducer of anthracycline biosynthesis from Streptomyces viridochromogenes. J Antibiot 1982;35:1722-1723 107. Hara O and Beppu T. Induction of streptomycin-inactivating enzyme by A-factor in Streptomyces griseus. J Antibiot 1982;35:1208-1215. 108. Yanagimoto M, Yamada Y and Terui G. Physiological study on the production of staphylomycin, 3: Extraction and purification of inducing material produced in staphylomycin fermentation. Hakko Kogecu Zassi 1979;57:6-19. 109. Kawagushi T, Asahi T, Satoh T, Uezumi T and Beppu T. B-factor an essential regulatory substance inducing the production of rifamycin in a Nocardia sp. J Antibiot 1984,37:1587-1595. 110. Szabó G, Bekeshi I and Vitalis S. Mode of action of factor C, a substance of regulatory function in cytodifferentiation. Biochem Biophys Acta 1967;145:159-165. 111. Scribner HE, Tang T and Bradley SG. Production of a sporulation pigment by Streptomyces venezuelae. Appl Microbiol 1973;25:873-879. 112. Novák J, Kopecký J and Vaněk Z. Sporulation-inducing factor in Streptomyces avermitilis. Folia Microbiol 1992;37:463-465. 113. Hirsch CF and Ensign JC. Some properties of Streptomyces viridochromogenes spores. J Bacteriol 1978;134:1056-1063. 114. Petersen F, Zaehner H, Metzger JW, Freund S and Hummel R-P. Germicidin, an autoregulative germination inhibitor of Streptomyce viridochromogenes NRRL B-1551. J Antibiot 1993;46:1126-1138. 115. Pospíšil S. Rezistance of Streptomyces cinnamonensis to butyrate and isobutyrate: production and properties of new ant-isobutyrate (AIB) factor. J General Microbiol 1991;127:2141-2146. 116. Janglová Z, Suchý J and Vaněk Z. Regulation of biosynthesis of secondary metabolites. VII. Intracellular adenosin-5´-triphosphate concentration in Streptomyces aureofaciens. Folia Microbiol 1969;14:208-210. 31 117. Čurdová E, Křemen A, Vaněk Z and Hošťálek Z. Regulation and biosynthesis of secondary metabolites, 18: Adenylate level and chlortetracycline production in Streptomyces aureofaciens. Folia Microbiol 1976;21:481-486. 118. Vu-Trong K, Bhuwapathanapun S and Gray PP. Metabolic regulation in tylosinproducing Streptomyces fradiae: Regulatory role of adenylate nucleotide pool and enzyme involvend in biosynthesis of tylonolide precursors. Antimicrob Agents Chemother 1980;17: 519-525. 119. Chatterjee DD and Vining LC. Nutrient utilization in actinomycetes: Induction of α-glucosidase in Streptomyces venezuelae. Can J Microbiol 1981;27:639-645. 120. Doul JL and Vining LC. In: Vining LC and Stuttard C (eds) Genetics and Biochemistry of Antibiotic Production, Buttereorth-Heinemann, Newton, 1995;9-64 121. Nigam P and Singh D. Solide-state (substrate) fermentation systems and their applications in biotechnology. J Basic Microbiol 1994;34:405-423. 122. Robinson T, Singh D and Nigam P. Solide state fermentation: a promising microbial technology for secondary metabolite production. Appl Microbiol Biotechnol 2001;55:284-289. 123. Niemi J, Ylihoko K, Hakala J, Parssinen R, Kopio A and Mansala P. Hybride anthracycline antibiotics: production of new anthracyclines by cloned genes from Streptomyces purpurascens in Streptomyces galilaeus. Microbiology 1994;140:13511358. 124. Semedo LTAS, Linhares AA, Gomes RC, Manfio GP, Aviano CS, Linhares LF and Coelho RRR. Isolation and characterization of actinomycetes from Brazilian tropical soils. Microbiol Res 2001;155:291-299. 32 Figures. Fig. 1. Fig. 2. Fig. 3. Fig. 4. Fig. 5. Fig. 6. Fig. 7. Fig. 8. Fig. 9. Fig. 10. Fig. 11. Fig. 12. Fig. 13. Fig. 14. Fig. 15. Fig. 16. Fig. 17. Fig. 18. Fig.19. Samples of streptomycetes mycelia were prepared according to standart procedures used for biological samples preparation for scanning electron microskopy. The samples were examined in Aquasem electron microscope (A) or in Philips CM12/STEM electron microscope (B,C and D) in STM mode. The samples were prepared and analysed in the Laboratory of Electron Microscopy of the Institute of Microbiology, Academy of Sicences, CR by Olga Kofroňová and Oldřich Benada. Bacitracin Bacitracin synthetase Penicillins Cephalosporins Clavulanic acid Vancomycin Tetracyclines Tetracycline biosynthesis Tetracycline biosynthesis Anthracyclines Erythromycins Tylosin and Relomycin Nystatins Avermectins A - R5= OCH3; B - R5= OH; 1 - X= -CH=CH-; 2 = X= -CH2-CHOH-; a - R26= C2H5; b - R26= CH3 Chloramphenicol Streptomycines 6-aminopenicillanic acid and 7-aminocephalosporanic acid Factor A, Factor B 33