Safe Management and WM2 comment /discussion document
advertisement

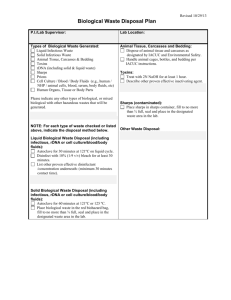
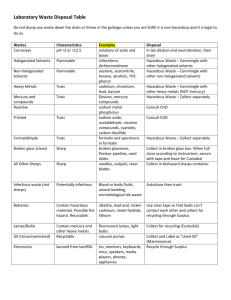
Safe Management and WM2 comment /discussion document The following represents of White Rose Environmental Ltd and are based upon Simple practical measures to help health practitioners comply with the legal requirements which It matches nursing practice so reducing the re-training/educational burden on the NHS Lowers the cost burden on the NHS Aligns requirements with ADR Provides real benefits to NHS and the environment through change The main questions raised by the consultation process: Q: Do you agree with the recommendation that clinical waste is redefined as hazardous infectious waste? If not, please give explanations? (Section 4.15) Comments No. Not all of the waste in the HSAC categories A-D is from known infectious agents. The majority of this waste is treated before landfill disposal on a precautionary basis. Hazardous infectious waste i.e. that requiring special precautions should be waste containing or suspected to contain those biological agents in o ADR category A or o Where particular practices are carried out i.e. barrier nursing that occur when there are known infection risks. Where barrier nursing is carried out then the waste should be regarded as being hazardous waste as special precautions are in place to avoid infection. This would allow the consignment of waste to follow to match nursing practice be easier to train and educate nursing staff to the required segregation practices required. This would allow other healthcare wastes to be treated before landfill on a precautionary basis but classed as non-hazardous. Q: Do you agree with the methodology proposed of identifying and classifying infectious and medicinal waste? If not, identify what alternative approach or methodology would be more acceptable? (Section 5.0) Comments The methodology proposed for infectious waste is a sensible approach to identifying vectors of transmission. If this methodology is used in conjunction with, as above, particular nursing practices i.e. barrier nursing, then this would enhance the assessment of the waste stream identifying clearly to producers those wastes that are hazardous. We would agree with the methodology for identifying hazardous medicinal products and non-hazardous medicinal products, however the route for disposal should also be made clear and this should be to incineration only. We also feel there should be no deminimus i.e. even a discharged syringe that has contained medicinal/chemical material should be sent for incineration. Q: Do you agree with the benefits of introducing an “offensive waste” stream? (Section 5.3) Comments A non-hazardous healthcare waste stream has always existed, HSAC category E waste. If the identification and consignment of hazardous waste is matched to ADR cat A groups and nursing practice i.e. when barrier nursing is instigated, then the majority of clinical waste i.e. that from general nursing practices would be regarded as non-hazardous. This has the cost advantage of reducing consignment costs to the NHS (estimated at £30-£40 mill per annum in the regulatory impact assessment to the Hazardous Waste Regulations consultation). The non-hazardous waste from general nursing practice should be treated before landfill on a precautionary basis. Q: Do you agree with the benefits of a nationally based system of colour-coded packaging? If not, please suggest any recommendations for an alternative approach? (Section 7.1) Comments Yes, provided it is not overly complicated. A national based system of colour-coded packaging is beneficial, however the suggested colour coding only allows for infectious hazardous and non-hazardous waste description. Further to comments above a colour code should be introduced to allow non-infectious waste to go to treatment before disposal. A suggested scheme is o Cytotoxic/cytostatic waste would be coded purple (18 0108) o Incineration only waste would be coded yellow (180101, 180102, 180106, 180107, 180109) o Waste for Treatment would be coded orange (treatment or incineration) (180103, 180104) o Waste going direct to landfill would be tiger stripe bag (180104) Q: Views are sought on the practicality of segregating sharps waste contaminated with cytotoxic/cytostatic medicinal products and sharps boxes not contaminated with cyto-medications. Suggestions are sought as to how waste products can demonstrate effective waste segregation. (Section 7.2) Comments The risk of improper segregation at Ward level is too great. All areas using Cytotoxic/Cytostatic should code all sharps as Hazardous waste. The document suggests that sharps are knives and scapel blades and syringe needles without the syringe body. Given current nursing practice for health and safety reasons it is impractical to suggest that syringe needles will be separated from syringe bodies after administering a medicine. Current practice is also to dispose of tubing/giving sets, spent ampoules, medicine bottles and tablet bottles and blister packs in the nearest receptacle after administering the drug; this is usually the sharps box. Given these practices it is not currently possible for hospital premises to practically segregate sharps waste. Given this information it is recommended that all sharps waste should go to incineration. If the definition given in the document for clinical waste were approved then as all sharps containers are pre-marked UN 3291 then all sharps waste would become hazardous waste by infection, regardless of the fact that they may contain chemical/medicinal products. Materials with infection risk are suitable for alternative treatment technologies; therefore it is reasonable that a large amount of sharps waste will find its way to alternative treatment plants. For this reason we would recommend that all sharps waste should be sent to incineration. Q: Do you have any other general comments you would like to make? Comments 1. This document introduces a binding definition of ‘rendered safe’ The definition and requirements given for ‘rendered safe’ would appear to be a significant enhancement above the guidance given in the EA document Technical Guidance on Clinical Waste Management facilities. It is considered that a DH guidance document is not the appropriate document for the EA to introduce new/significantly revised guidance i.e. mandatory pre- or integral shred. The definition of rendered safe in the Ea Technical Guidance on Clinical Waste Management Facilities includes the footnotes that the waste need not be shredded if anatomical or sharps are not present in the waste. If the segregation/colour coding practices described earlier are followed i.e. all sharps to incineration, anatomical to incineration then the need for treated waste to be shredded disappears. Where pre- or integral shred is mandated by the EA, this introduces an activity into the workplace at a treatment site that will inherently have infection risks during equipment breakdown or maintenance, especially if the malfunction is to the cleaning / disinfections process. Current Health & Safety Executive policy through risk assessment is to eliminate risks first, however through the practice of mandatory preintegral shred the EA does not allow the operator the opportunity to eliminate the risk and so brings the operator in conflict with health & safety legislation and regulatory practice. The timescale and dates given for introducing the requirement to ‘pre or integral shed waste at alternative treatment sites needs to be revised. The document does not appear to allow any alternatives to preshredding for instance segregation. The desire for pre-shred appears to originate following discovery of medical devices that withstand the treatment cycle in alternative technologies. As segregation of other waste streams not considered suitable for alternative treatment is considered satisfactory e.g. radioactive waste, anatomical waste segregation of specific medical devices would also seem appropriate. The segregation of these devices and other devices/containers could be made more successful if they were ‘source tagged’ with either an electromagnetic or RFID tag and a detector installed at the treatment site. The use of this type of RFID technology is well established in the retail industry and so is available, low cost and extremely reliable. Practice at the treatment site would be that once the detector alarms then waste would be segregated for alternative disposal e.g. incineration. This technology solution could also be employed to source tag other medical devices or containers e.g. cytotoxic sharps bins that should not find their way into non-incineration sites. As the document includes references to procurement practices for waste management contracts, procurement contracts for medical devices should also include information on manufacturers ensuring there is an appropriate disposal route for their waste/devices. Where the disposal required a particular treatment e.g. incineration then source tagging or identification for segregation should become part of the marking or design of the device. 2. Anatomical Waste The document suggests that all anatomical waste be regarded as infectious. Not all anatomical waste is infectious e.g. waste arising from transplant surgery, cosmetic surgery, nor should it automatically be regarded as so. 3. Storage Periods The document suggests that the clinical waste should be stored for 7 days unless refrigerated, we feel this is onerous and storage periods are not required where waste is held in suitable, sealed containers. 4. Sector Guidance Documents The guide states that sector guides would be produced. We welcome this and see these documents providing the practical guidance to practioners and health professionals missing from the parent document, however we would state there would need to be clear timescales by when these are to be published and these should be very soon after the publication of the parent document.