SOP-M004 Mouse Intracellular Cytokine Staining
advertisement

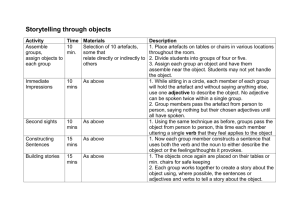
Author Date Version SOP # Stephanie Swift 16th July 2015 4 M004 Objective: This protocol addresses the processing, peptide stimulation and antibody staining of fresh mouse peripheral blood for flow cytometric analysis M004 - Intracellular Cytokine Staining Required reageants and equipment: ACK lysis buffer (see Appendix A) 1x heparin (see Appendix A) (Sigma #H3149) 5% FCS (25ml FCS + 475ml PBS) FACS Buffer (2.5g BSA in 500ml PBS) cRPMI (RPMI + 10% FCS + L-Glut + P/S + HEPES) Golgi Plug (BD Biosciences #554715) Cytofix-Cytoperm (BD Biosciences #554715) Permwash buffer (BD Biosciences #554715; 50ml stock in 450ml dH2O) CD8-PerCPCy5.5 (BD Biosciences clone 53-6.7) IFNg-APC (BD Biosciences clone XMG1.2) TNFa-FITC (BD Biosciences clone MP6-XT22) IL-2-PE (BD Biosciences clone JES6-5H4) Viability Dye-eF450 (eBiosciences #65-0863-14) 96-well U-bottom plates Phorbol 12-myristate 13-acetate (PMA) (Sigma #P-1585) Ionomycin (Sigma #I-0634) One Comp eBeads (eBioscience #01-1111-42) PBS A. Processing Blood Samples Blood samples are collected from a non-terminal saphenous bleed (~5 drops per mouse into a tube containing 200ul 1 x sterile heparin). Always collect blood from two extra naive mice for your negative (no peptide) and positive (PMA/ionomycin) controls (plus unstained compensation control). 1. Measure the total volume of blood (remember to subtract the 200ul 1 x heparin collection solution) collected for each individual mouse, and note down in labbook. 2. Add 2ml ACK to each tube, flick to resuspend and incubate 5 mins. at RT. 3. Add 6ml PBS to wash. Spin down at 1500RPM for 5 mins. at 4C. 4. Pour off supernatant, resuspend by flicking. Note. If the pellet is larger than 2mm, proceed on to a second ACK lysis (step 5). Otherwise, proceed straight on to the cRPMI wash in step 7. 5. and add 1ml ACK. Flick to resuspend and incubate 3 mins. at RT. 6. Add 6ml PBS to wash. Spin down at 1500RPM for 5 mins. at 4C. 7. Pour off supernatant, add 2ml cRPMI to wash. Spin down at 1500RPM for 5 mins. at 4C, remove supernatant & resuspend by flicking. 8. Resuspend in a volume of cRPMI that allows you to plate 100ul per well of a 96-well U-bottom plate, taking into account that there will be ~ 50ul left in the tube after decanting the final cRPMI wash. Ideally, plate 100ul for an unstimulated control and 100ul for a stimulated control per mouse. This protocol is shared by the Stojdl Lab under a creative commons license. Note. If your pellets were small, or you have to split your non-terminal blood sample into more than two wells, use 50ul for your unstimulated well, or don't include an unstimulated and simply stimulate blood from a naive mouse with your peptide(s) of interest as your control. Try to limit splitting of non-terminal bleed samples to no more than 4 aliquots due to low cell numbers. B. Peptide Stimulation 9. Add 50ul/well of peptide(s), diluted in cRPMI, to each sample. See Appendix B for example peptides. e.g. for SIINKFEKL, final concn in well = 1ug/ml. Stock = 1mg/ml (0.2ul/well). For negative (unstimulated) controls, add 50ul/well of DMSO diluted in cRPMI at the highest concentration used in your test peptide stimulations (see Appendix B). For positive controls, add 50ul/well of PMA/ionomycin (see Appendix B for dilutions). Note. For weak peptides, further stimulation may be necessary with antiCD28 - 0.1ul per sample; reduce cRPMI volumes accordingly. 10. Incubate for 1h at 37C. 11. After 1h, add 50ul diluted Golgi Plug to both unstimulated and stimulated wells. See below for example calculation for 20 wells:- (per sample, always make extra to account for pipetting loss/error) Reageant Golgi Plug cRPMI Dilution 0.2ul per well - Volume (ul) 4 996 12. Incubate for a further 4h at 37C. 13. Spin down at 1500RPM for 5 mins. at 4C. If you wish to leave cells overnight at this point, resuspend cells in 200ul 5% FCS. Store at 4C o/n. If you wish to continue immediately to staining, wash cells twice in 200ul FACS buffer. C. Intracellular Cytokine Staining (ICS) Note: The dilutions of each antibody may change between different lots of the same antibody clone received from the same company. Individual lot titrations should be carried out to optimise dilutions. 14. Spin down plate and tip off supernatant. 15. Add 50ul diluted Fc block per well, mix and incubate for 15 mins. on ice. See below for example calculation for 20 wells:- (per sample, always make extra to account for pipetting loss/error) Reageant Fc block FACS Buffer Dilution 1:100 - Volume (ul) 10 990 16. Add 150ul FACS buffer to wash, spin down at 1500RPM for 5 mins. at 4C, and tip off supernatant. 17. Add 50ul/well of diluted CD8 and/or CD4 antibody together with live/dead viability stain, mix and incubate for 30 mins. on ice IN THE DARK. See below This protocol is shared by the Stojdl Lab under a creative commons license. for example calculation for 20 wells:- (per sample, always make extra to account for pipetting loss/error) Reageant CD8-FITC Viability Dye-eF450 FACS Buffer Dilution 1:400 0.1ul per well - Volume (ul) 2.5 2.0 995.5 18. Wash 150ul FACS buffer, spin down at 1500RPM for 5 mins. at 4C, and tip off supernatant. 19. Wash 200ul FACS buffer, spin down at 1500RPM for 5 mins. at 4C, and tip off supernatant. 20. Fix with 100ul Cytofix-Cytoperm, mix well and incubate for 20 mins. on ice IN THE DARK. 21. Wash in 100ul permwash buffer, spin down at 1500RPM for 5 mins. at 4C, and tip off supernatant. 22. Wash in 200ul permwash buffer, spin down at 1500RPM for 5 mins. at 4C, and tip off supernant. Pellets should now be transparent. 23. Add 50ul diluted intracellular antibody cocktail for 30 mins. on ice IN THE DARK. See below for example calculation for 20 wells:- (per sample, always make extra to account for pipetting loss/error) Reageant IFNg-APC TNFa-PECy7 IL-2-PE Permwash buffer Dilution 1:100 1:200 1:100 - Volume (ul) 10 5 10 975 24. Add 150ul permwash buffer, spin down at 1500RPM for 5 mins. at 4C, and tip off supernatant. 25. Add 200ul permwash buffer, spin down at 1500RPM for 5 mins. at 4C, and tip off supernatant. 26. Final resuspension in 200ul FACS buffer. Keep cells on ice and in the dark until running on flow cytometer. Note: for quantitative analysis (relative #), #’s obtained from FlowJo analysis will have to be adjusted to account for differences in the total volume in each blood sample prior to processing. See appendix C. To prepare compensation controls:1. Vortex compensation beads (eBioscience One Comp eBeads #01-1111-42). Label a separate tube for each antibody/fluorophore used in your experiment. 2. Add 1 drop beads per tube. 3. Add 1ul of antibody stock. Vortex. Incubate at RT for 15-30 mins. IN DARK. 4. Add 2ml FACS buffer, pellet by centrifugation at 1500RPM for 5 mins. Pour off supernatant. 5. Resuspend bead pellet in 300ul FACS buffer. Vortex. This protocol is shared by the Stojdl Lab under a creative commons license. APPENDIX A ACK Lysis Buffer 8.29g Ammonium Chloride 1g Potassium Bicarbonate 200ul 0.5M EDTA pH8.0 800ml dH2O pH to 7.4, then make up to 1L with dH2O 10x Sterile Heparin (1x working stock) The 10x sterile heparin stock solution should be at 400U/ml. e.g. weigh 122mg heparin sodium salt (from porcine intestinal mucosa) at 164 USP units/mg. r/s in 50ml PBS = 400U/ml solution. Filter stock solution through 0.45um filters. This can be stored for several months at 4 degrees Celsius. The working stock (to collect blood into) should be diluted 10-times to 40U/ml in PBS. Can store the working stock for several months at 4 degrees Celsius. This protocol is shared by the Stojdl Lab under a creative commons license. APPENDIX B Preparing Negative Control Master Mix Add 50ul/well of DMSO diluted in cRPMI at the highest concentration used in your test peptide stimulations e.g. if you stimulated some wells with 1ul SIINFEKL peptide in 5 ml cRPMI, and some wells with 10uL m38 peptide in 5 ml, make up your negative control by adding 10ul of DMSO to 5ml cRPMI. Preparing Positive Control PMA/Ionomycin Master Mix 1. Phorbol 12-myristate 13-acetate (PMA) powder (Sigma #P-1585) a. Reconstitute in DMSO at 0.1 mg/ml. b. Store small aliquots (eg, 20 uL) at –20°C; do not refreeze aliquots after thawing. c. Dilute 1:100 in sterile PBS (without sodium azide) for each experiment to prepare a fresh stock solution. 2. Ionomycin powder (Sigma #I-0634) a. Reconstitute in EtOH at 0.5 mg/ml. b. Store at –20°C. c. Dilute 1:10 in sterile PBS (without sodium azide) for each experiment to prepare a fresh stock solution. 3. Mix fresh PMA solution at a final concentration of 10 ng/ml of cell suspension (0.5ul per 50ul - in cRPMI) with fresh ionomycin solution at a final concentration of 1ug/mL of cell suspension (1ul per 50ul - in cRPMI). See example calculation below for 20 wells:Reageant PMA (1:100 fresh) Ionomycin (1:10 fresh) cRPMI Volume (ul) 10 20 970 Preparing Test Peptide Master Mix Check stock concentration of peptides – you may need to adjust the following calculations for different peptides. Default final peptide concentrations are 1ug/ml. CD8+ Epitopes in H-2b haplotype (i.e. C57BL/6): Peptide MHC H2-Kb H2-Db H2-Db H2-Kb H2-Kb H2-Kb H2-Kb H2-Kb H2-Kb Protein hDCT180-188 gp10025-33 gp10025-33 OVA257-264 βgal97-104 VSV N52-59 FMT N301-309 MCMV m38316-323 AdV DBP418-426 Peptide Sequence SVYDFFVWL KVPRNQDWL EGSRNQDWL SIINFEKL DAPIYTNV RGYVYQGL AVVLMFAQC SSPPMFRV FALSNAEDL Stock Final Concentration Concentration 5mg/ml 10mg/ml 10mg/ml 5mg/ml 5mg/ml 10mg/ml 2ug/ml 1ug/ml 1ug/ml 1ug/ml 1ug/ml 1ug/ml 1ug/ml 10ug/ml 20ug/ml CD8+ Epitopes in H-2d Haplotype (i.e. Balb/C): Peptide MHC Protein Peptide Sequence Stock Final Concentration Concentration This protocol is shared by the Stojdl Lab under a creative commons license. CT26 Tumour Cells H2-Dd VSV N275-283 SPSYVYHQF MPYLIDFGL 1ug/ml 1ug/ml This protocol is shared by the Stojdl Lab under a creative commons license. APPENDIX C Converting Frequencies to Absolute Numbers If the volume of blood collected from each mouse is known, PBS-treated control mice were included in the experiment and samples were processed in parallel and run through the flow cytometer in the same final volume for exactly the same length of time, then frequencies can be converted to physiologically-relevant absolute #’s as follows: 1. Add up the total # of lymphocyte events collected on the flow cytometer. Calculate the mean # of lymphocyte events collected from the PBS control group. 2. Calculate the adjustment factor that converts the mean # of lymphocyte events to the expected physiological # (=7.07x103/μl blood; Charles River Laboratories dataset for female C57BL/6 mice; BALB/c=6.69x103/μl blood). Multiply all raw lymphocyte numbers by this adjustment factor. 3. All lymphocyte subset frequencies can then be multiplied by the adjusted lymphocyte # to determine their respective #’s. To estimate total # of cells circulating in the mouse (i.e. scale up from your nonterminal blood volume to the total blood volume of the mouse), assume each mouse has a total blood volume of 1.89 ml (based on the weight chart for female C57BL/6 mice provided by Charles River Labs and “Methods of blood collection in the mouse” J. Hoff, Lab Animal, 29:10, pp. 47-53; assumptions: an average age of 10 weeks at the time of sampling, a mean body weight of 21 g and a mean blood volume of 9 % of body weight). This protocol is shared by the Stojdl Lab under a creative commons license. APPENDIX D - Cell Phenotype Markers T Cells Myeloid Dendritic Cells CD3+ CD4+ and/or CD8+ CD11b+ CD11chi Regulatory T Cells B220- CD3+ CD4+ Gr-1NK1.1- Foxp3+ CD3- CD25+ MHC class II+ CTLA4+ Macrophages/Monocytes GITR+ CD127lo Gr-1CD11blo/mid B Cells CD11c+ B220+ CD19+ B220F40/80+ CD3- NK1.1- NK Cells CD3- CD3- MHC class II+ NK1.1/DX5+ Myeloid Suppressor Cells Plasmacytoid Dendritic Cells Gr-1+ CD11blo/mid CD11cmid CD11b+ CD80+ B220+ CD40- Gr-1+ B220- NK1.1- CD86- IKDC’s CD3MHC class CD19- II+ B220+ CD11c+ NK T Cells MHC class II+ CD3+ NK1.1/DX5+ NK1.1/DX5+ CD3Gr-1- This protocol is shared by the Stojdl Lab under a creative commons license.