H7N9 Influenza: Important interim information for
advertisement

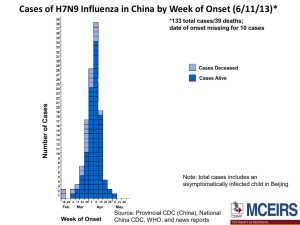
H7N9 Influenza: Important interim information for Medical Officers of Health, Clinicians and Laboratories Updated 29 July 2013 Key changes Updated case numbers Notification of H7N9 influenza as non-seasonal influenza under the Health Act 1956 as from 1 August 2013 Summary Between 31 March and 20 July 2013, 134 cases of influenza A(H7N9), including 43 deaths, have been reported in China, with the onset date of the last case on 10 July (updated numbers are available on WHO website at http://www.who.int/influenza/human_animal_interface/influenza_h7n9/en/index.html). Most reported cases with confirmed H7N9 infection have occurred in middle-aged or older men with underlying chronic conditions. Cases presented with pneumonia, with most of these patients being severely ill. There is currently no evidence of ongoing human to human transmission. Although the environmental source has not yet been definitively determined, most of the confirmed cases are reported as having had a history of recent exposure to poultry, generally in a live animal “wet market” environment. In patients with severe acute respiratory illness with a history of travel to China, or contact with known confirmed or probable cases of H7N9 influenza, within two weeks of illness onset, the following is recommended: 1. Place the patient in a single room with negative pressure air-handling, or a single room from which the air does not circulate to other areas, and implement standard and transmission-based precautions (contact and airborne), including the use of personal protective equipment (PPE). 2. Investigate and manage the patient as for community acquired pneumonia. Appropriate specimens should also be collected for influenza PCR testing. 3. Notify any suspected, probable or confirmed cases promptly to the local Medical Officer of Health. From 1 August, H7N9 is notifiable by medical practitioners (on suspicion) and laboratories as “nonseasonal influenza” under the Health Act 1956. What is H7N9 influenza? Influenza A (H7) viruses are a group of influenza viruses that normally circulate among birds. A(H7N9) is a novel reassortant virus derived from three different avian influenza viruses. This strain is distinct from the A(H1N1)pdm09 (that caused the 2009 pandemic in humans) and A(H5N1) influenza. H7N9 that is genetically similar to that detected in infected humans has been detected in pigeon, poultry and environmental samples collected at a live animal market in Shanghai. Unlike the highly pathogenic avian influenza H5N1 virus, this new virus appears to be a low pathogenic avian virus and is hard to detect in poultry as it causes little to no signs of disease. 1 There is no evidence of sustained human-to-human transmission. Monitoring and testing of over 2000 contacts of confirmed cases has detected few infections. However, some small clusters (of two or more people) suggest that limited human-to-human transmission may occur when there is close contact between cases and other people. Moreover, genetic analysis indicates the virus has properties to infect mammalian cells; therefore, the potential for avian-human and human-human transmission exists but requires further investigation. Sequences previously associated with high virulence of A(H7) in humans (PB2 gene) have been detected in isolates in the current outbreak. What is the current situation? Please refer to WHO website on the current situation, including epidemiological updates, Q&A and guidance documents: Disease Outbreak News http://www.who.int/csr/don/en/index.html Avian influenza A(H7N9) virus http://www.who.int/influenza/human_animal_interface/influenza_h7n9/en/index.html Influenza at the Human-Animal Interface http://www.who.int/influenza/human_animal_interface/en/. Has WHO recommended any travel or trade restrictions related to this new virus? WHO does not advise special screening at points of entry with regard to this event, nor does it advise the application of any travel measures with respect to visitors to or from China. There is no evidence to link the current cases with any Chinese products. WHO advises against any restrictions to trade at this time. What are the symptoms? H7N9 was initially identified in patients with severe pneumonia and/or Acute Respiratory Distress Syndrome (ARDS), though there have been some confirmed mild cases. Symptoms have included fever ≥ 38C, cough, shortness of breath and evidence of lower respiratory tract disease. Complications of H7N9 virus infection have included septic shock, acute respiratory distress syndrome and respiratory failure , and multiple organ dysfunction. Symptoms and signs of A(H7) infections during previous outbreaks have mainly been conjunctivitis and mild upper respiratory symptoms, with the exception of one death, which occurred in the Netherlands. Are health workers at risk from H7N9 influenza? The routes of transmission to humans of the H7N9 influenza have not yet been fully determined, but there is currently no evidence of sustained human-to- human transmission. Infection control recommendations in this document for suspected, probable and confirmed cases aim to provide the highest level of protection for health care workers, given the current limited state of knowledge. Health care workers should follow local infection control guidelines for precautions against airborne infections. Who do I test for H7N9 influenza? Testing should be considered for: 1. Individuals with severe acute respiratory infection and history of travel to, or residence in China within the previous two weeks. 2 2. Individuals with severe acute respiratory infection and history of contact with those in point 1 above. 3. Health care workers with severe acute respiratory infection who have been caring for patients with severe acute respiratory infections, particularly patients requiring intensive care and with travel, residence or contact history as in points 1 and 2 above. 4. Clusters1 of unexplained severe acute respiratory infections. Please contact your local Medical Officer of Health to notify any suspected cases. How do I test for H7N9 influenza? Samples from patients with severe acute respiratory illness, suspected to be H7N9, should be referred via regular laboratory transport network systems to one of the virology referral laboratories (a list is provided in the Annex). Specimens should be double bagged. They should be clearly identified as requiring urgent testing for influenza A/H7N9, and separated from non-urgent specimens. The referral laboratory should be notified. Gloves, gown, P2 mask and eye protection should be worn when collecting samples from patients. If a negative pressure room is unavailable, the patient should be placed in a single room with the door closed. Collect two separate samples, a nasopharyngeal swab (optimal from both adults and children) and a throat swab or nasopharyngeal aspirate (usually from children), and place in viral transport medium. Sputum is strongly recommended wherever possible. Bronchoalveolar samples and lung biopsy should also be sent if available. Testing for influenza and other infectious causes should be undertaken at the referring laboratory using enhanced PC2 precautions and PPE, including a surgical mask and eye protection, and processing samples in a biosafety cabinet. Routine tests for acute pneumonia should be performed where indicated, including bacterial culture, serology, urinary antigen testing and tests for other respiratory viruses. Laboratories should immediately refer all unsubtypeable influenza A virus, all influenza A not subtyped, all H7N9 specimens and the original specimen to the WHO National Influenza Centre, i.e. ESR (NCBID). Confirmatory testing at a WHO laboratory is a requirement under the International Health Regulations for reporting H7N9 influenza cases to WHO. Any laboratory with a positive H7N9 should immediately notify the local Medical Officer of Health. PC3 conditions are required for virus culture. Rapid/Point of care diagnostic tests for influenza A & B are NOT recommended because of their lack of sensitivity. 1 A “cluster” is defined as two or more persons with onset of symptoms within the same 14 day period and who are associated with a specific setting, such as a classroom, workplace, household, extended family, hospital, other residential institution, military barracks or recreational camp. 3 Further details about specimen shipping and the contact details of virology laboratories are provided in the Annex. Additional laboratory information is available in National Laboratory Guidelines for Pandemic Influenza (currently under review) at http://www.health.govt.nz/publication/national-laboratory-guidelines-pandemic-influenza. What are the recommended isolation and PPE recommendations for patients in hospital? While further information is accumulating, current recommendations are for airborne transmission precautions for suspected, probable or confirmed cases. These recommendations on isolation and PPE for suspected, probable and confirmed cases take a deliberately cautious approach by recommending measures that aim to control the transmission of pathogens that can be spread by the airborne route. In summary, transmission-based precautions for suspected, probable and confirmed cases should include: Placement of cases in a negative pressure room if available, or in a single room from which the air does not circulate to other areas Airborne transmission precautions, including routine use of a P2 respirator, disposable gown, gloves, and eye protection when entering a patient care area Standard and contact precautions, including close attention to hand hygiene If a single or negative pressure room is not available (e.g. in primary care settings), or if transfer of the confirmed or probable case outside the negative pressure room is necessary, the patient should wear a surgical face mask, if tolerated, while they are being transferred and to follow respiratory hygiene and cough etiquette. Triage areas should have signs asking that patients with severe respiratory tract infections with a recent history of travel to China should make themselves known so that appropriate arrangements can be made. 4 Case Definitions 1. Suspected case (under investigation) A person with a Severe Acute Respiratory Infection (SARI)* AND with one or more of the following exposures during the two weeks prior to the onset of symptoms: - Travel to a country† where human cases of H7N9 influenza have recently been reported, especially if there was recent direct or close contact with animals (e.g. wild birds, poultry or pigs). - Close contact‡ with a laboratory-confirmed case. 2. Probable Case A person fitting the definition of a suspected case but with no possibility of laboratory confirmation for H7N9 influenza, either because the patient or samples are not available for testing AND not already explained by any other infection or aetiology, including all clinically indicated tests for community acquired pneumonia according to local management guidelines. 3. Confirmed Case A person with laboratory confirmation of infection with H7N9 influenza at the WHO National Influenza Centre (ESR, NCBID). * An acute respiratory illness with a history of fever or measured fever of ≥38°C, AND cough, AND onset within the past two weeks, AND requiring inpatient hospitalisation (defined as a patient who is admitted under a medical team and to a hospital ward or assessment unit). † Currently, China (excluding Hong Kong) is the only country that has recently reported human cases of H7N9 influenza. ‡Close contacts include: any person who provided care for the patient or who had other similarly close physical contact in the two weeks before symptom onset; this includes health care workers or family members. any person who stayed in the same place (e.g. household, office) as a probable or confirmed case while the case was symptomatic. Note: Although most of the cases to date have presented with a severe acute respiratory illness, mild cases have been reported. If doctors are concerned about patients presenting with milder illness, they should discuss this with the local Medical Officer of Health. The Medical Officer of Health will perform an initial risk assessment to determine whether the individual meets the case definition and if so will discuss next steps with the Ministry of Health. 5 Notification As of 1 August 2013 Influenza A(H7N9) is notifiable as “non-seasonal influenza” (Schedule 1 of the Health Act 1956). As such, any suspected case should immediately be notified to the local Medical Officer of Health, either by the attending medical practitioner or laboratory. Any contacts of a case (including a suspected case) should also be reported to the local Medical Officer of Health. Any change in a case status (e.g. case confirmation, death or de-notification) should also be immediately reported. WHO will be notified of probable and confirmed cases through the National Focal Point for International Health Regulations (i.e. the Office of the Director of Public Health, Ministry of Health). Advice for contacts of cases Contacts of cases should be directed to the local Medical Officer of Health for advice. The local Medical Officer of Health will undertake an initial risk assessment to determine whether the individual is likely to be a contact and advise on appropriate contact management measures (such as quarantine). Advice for travellers to China At this time, it is advisable that travellers to China keep away from poultry and livestock and avoid visiting live “wet” animal markets. Advice for returned travellers At this time, if returned travellers meet the exposure criteria for the case definition but have a less severe respiratory illness, advice regarding further management should be sought from the local Medical Officer of Health. The local Medical Officer of Health will undertake an initial risk assessment to determine whether the individual is likely to be a case or a contact. Other useful links UN Food and Agriculture Organization of the United Nations (FAO) http://www.fao.org/news/story/en/item/173655/icode/ 6 Annex – Laboratory Response When specimens from suspected A(H7N9) cases are collected, ring immediately one of the referral laboratories listed below for testing for influenza viruses: ESR: WHO National Influenza Centre Institute of Environmental Science and Research National Centre for Biosecurity and Infectious Disease 66 Ward Street, Wallaceville, Upper Hutt 5018 Phone: (04) 529 0606 After hours (Mobile): 027 216 7833 Auckland (ADHB): Department of Virology LabPLUS Building 31 Auckland City Hospital PO Box 110 031 Grafton, Auckland Phone: (09) 307 8995 After hours: (09) 379 7440 Auckland (CMDHB): Microbiology Department Middlemore Hospital PO Box 93311 Otahuhu Manukau 1640 Phone: (09) 276 0044 Waikato region: Specialist Services Laboratory Waiora Building Level 3 Waikato Hospital Pembroke Street Private Bag 3200 Hamilton Phone: 0800 452 283 ext 8530 (Virology) After hours: Pager 20075 Wellington region: Laboratory Services Clinical Services Block, Level F Wellington Hospital Riddiford Street, Newtown Private Bag 7902 Wellington Phone (including after hours): (04) 385 5999 ext 6060 Southern region: Virology/Serology Section, Microbiology Department, Canterbury Health Laboratories, PO Box 151, Christchurch. Ph: (03) 364-0356 (Virology lab) Ph: (03) 364-1229 (Section Head) After hours: (03) 364 0640 7 PROCEDURE FOR SHIPPING RESPIRATORY SAMPLES TO WHO National Influenza Centre at ESR NCBID ESR complies with land and air transport regulations (UN3373 Category B-Biological Substance*) for transporting human respiratory samples. (*Note: Dangerous Goods form is NOT required.) How to send your samples: 1. Place the vial containing VTM and the respiratory swab into its own specimen biohazard bag and seal the zip lock bag. Then place the completed specimen request form (including practice name) into the outer pocket of the specimen bag for that sample. 2. Please keep samples refrigerated until they can be couriered to ESR NCBID. 3. Place sample bag(s) into the bubble wrap bag with absorbent pads (the two absorbent pads are there to soak up any spill). Then place the package into the orange lidded Bio-bottle and add the frozen icepacks (in their own plastic bag). Close the lid tightly. 4. Place the Bio-bottle into its outer cardboard box and close the top. Check the address cards around on the outer cardboard box are the correct way around (i.e To: ESR NCBID, From: Your Practice address). The ESR address is: ESR NCBID – Wallaceville, 66 Ward Street, Wallaceville, Upper Hutt 5018. 5. Attach the supplied New Zealand Courier ticket onto the box and phone 0800 800 841 to arrange pick-up of the container. Respiratory Sample Kit The full kit consists of: 10 x vials containing viral transport medium; 10 x swabs; 10 x plastic specimen bags; 10 x specimen request forms; 10 x return courier tickets; 1 x plastic bubble bag; 2 x adsorbent pads; 3 x ice packs in plastic bags (keep frozen until ready to use); 1 x plastic Bio-bottle, 1 x ESR Bio-bottle outer cardboard box with sampling and shipping instruction sheet attached. (Note: the number of swabs/VTM can be varied depending on the situation.) For supply of more VTM, swabs, Bio-bottles and specimen request forms and courier tickets, please contact: o ESR NCBID - Wallaceville Specimen Reception, Phone (04) 529 0604, Fax (04) 529 0601 or o Judy Bocacao at the WHO National Influenza Centre at ESR NCBID - Wallaceville Phone (04) 529 0618 ESR will return Bio-bottles to the sender by courier throughout the surveillance season. Please return unused courier tickets, Bio-bottles, VTM etc to ESR NCBID at the end of the surveillance programme 8 Laboratories should immediately refer all unsubtypeable influenza A virus, all influenza A not subtyped, all H7N9 specimens and the original specimen to the WHO National Influenza Centre (NIC), i.e. ESR (NCBID). The main reason is that confirmatory testing at a WHO laboratory is a requirement under the International Health Regulations for reporting H7N9 influenza cases to WHO. There are additional significant reasons: H7N9 is a new assay, and its clinical performance has not been fully evaluated. Confirmation testing at the NIC in early cases will give confidence in the assay performance. Additional testing can be done at the NIC, such as oseltamivir susceptibility testing and next generation sequencing. The NIC acts as repository for all NZ virus influenza isolates, and is the national focal point for international sample sharing (in particular with WHO Influenza Collaborating Centre in Melbourne as well as CDC in Atlanta). If required, the NIC can produce H7 virus in PC3 laboratory to be used in other essential assays such as neutralisation if required. 9