metals atoms
advertisement
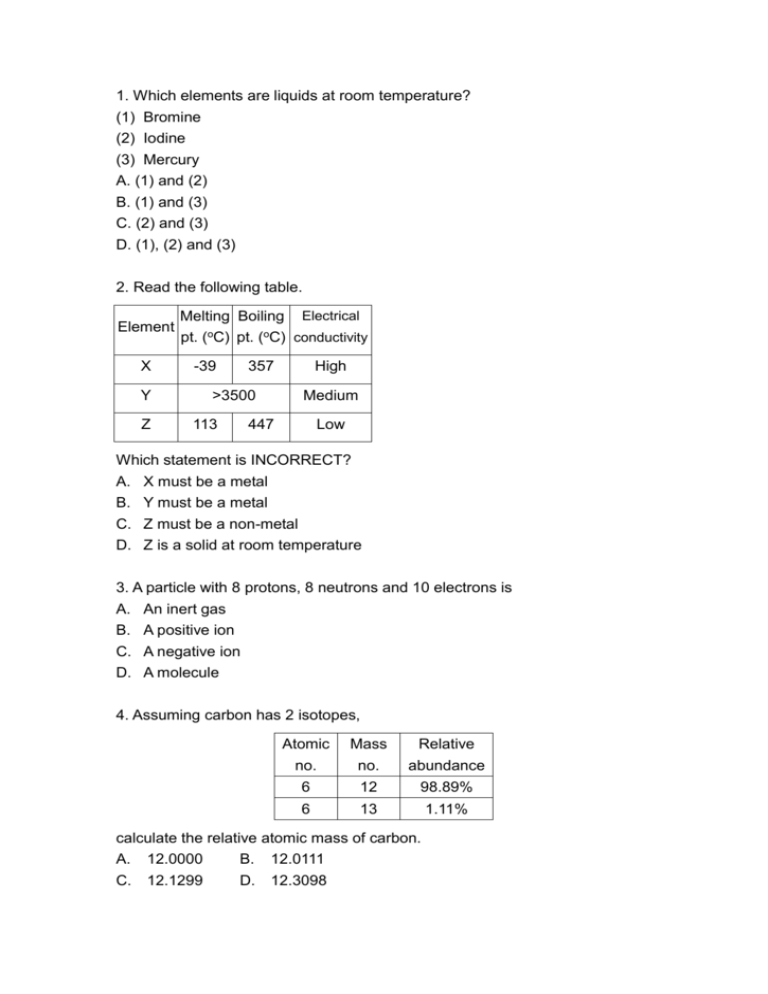
1. Which elements are liquids at room temperature? (1) Bromine (2) Iodine (3) Mercury A. (1) and (2) B. (1) and (3) C. (2) and (3) D. (1), (2) and (3) 2. Read the following table. Element X Y Z Melting Boiling Electrical pt. (oC) pt. (oC) conductivity -39 357 >3500 113 High Medium 447 Low Which statement is INCORRECT? A. X must be a metal B. Y must be a metal C. Z must be a non-metal D. Z is a solid at room temperature 3. A particle with 8 protons, 8 neutrons and 10 electrons is A. An inert gas B. A positive ion C. A negative ion D. A molecule 4. Assuming carbon has 2 isotopes, Atomic no. Mass no. Relative abundance 6 12 98.89% 6 13 1.11% calculate the relative atomic mass of carbon. A. 12.0000 B. 12.0111 C. 12.1299 D. 12.3098 5. Which of the following pairs of atoms would have similar chemical properties? A. 6X and 15Y B. 4X and 20Y C. 9X and 16Y D. 7X and 17Y 6. Which of the following groups of elements are metals? (1) Group I (2) Group II (3) Group 0 A. (1) and (2) B. (1) and (3) C. (2) and (3) D. (1), (2) and (3) 7. Which of the following substance is NOT a mixture? A. Distilled water B. Mineral water C. Sea water D. Rain water 8. Which properties are common to all metals? (1) High electrical conductivity (2) High heat conductivity (3) Denser than water A. (1) and (2) only B. (1) and (3) only C. (2) and (3) only D. (1), (2) and (3) 9. Metals are ductile and malleable because A. Layers of metal atoms can slip over one another. B. Metallic bonds are very strong C. Metal atoms are attracted to each other by strong forces. D. Metals atoms are closely packed in the solid state. 10. Which statement is correct for a metal that is found in the free state in nature? A. The metal must be very expensive B. The metal must be inert towards water and air C. The compounds of the metal must be soluble. D. None of the above statement is correct. 11. Which of the following is NOT is a way of conserving metals? A. Reuse metals B. Replace metals with other materials such as plastics C. Recycle metals from used-metal wastes. D. Increase the number of known metal ore locations. 12. The advantages of recycling metal are due to (1) Reducing the demand for metal ores (2) Saving energy (3) Reducing pollution from metal waste and mining A. (1) and (2) only B. (1) and (3) only C. (2) and (3) only D. (1), (2) and (3) 13. The cost of recycling metals include: (1) Melting and purifying the metal (2) Collecting the scrap metal (3) Disposing the waste from the recycling process A. (1) and (2) only B. (1) and (3) only C. (2) and (3) only D. (1), (2) and (3) 14. Brass but not pure copper is used for making door knobs because it (1) Is more corrosion resistant (2) Is harder (3) Is more malleable A. (1) only B. (1) and (2) only C. (1) and (3) only D. (2) and (3) only 15. Which statement concerning iron is INCORRECT? A. Steel is an alloy of iron and carbon B. Mild steel is usually used for large objects such as car bodies C. Stainless steel is harder and stronger than pure iron D. Stainless steel is usually used for making small objects such as cutlery. 16. Emulsifiers are added to foods to A. Enhance the flavour B. Make oil and water mix to form an emulsion C. Prevent oil and water from mixing D. Prevent discolouration. 17. Which of the following drug is NOT a pain-killer? A. Paracetamol B. Morphine C. Aspirin D. Nicotine 18. Which of the following substance can act as a food preservative by dehydrating the food and micro-organism? A. Salt B. Vinegar C. Benzoic acid D. Sulphur dioxide 19. Disease induced by smoking include (1) Lung cancer (2) Coronary heart disease (3) (4) A. B. C. D. Bronchitis Flu (1) and (2) (3) and (4) (1), (2) and (3) only (1), (2), (3) and (4) 20. What is the main reason why smokers find it hard to give up smoking? A. Smoking is a pleasant social habit B. Smokers are addicted to nicotine C. Smokers are influenced by cigarette advertisement. D. Giving up smoking is a sign of cowardice