EXPERIMENTAL AND MODELLING STUDY OF PURE
advertisement

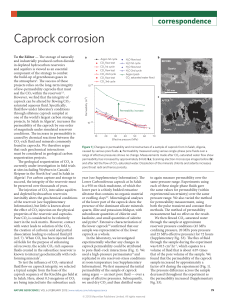
Contribution à : ARE »CAP ROCKS« SAFE SEALS FOR CO2? Intro-Conclusion-Synthèse: (J. Pironon et G. Hubert) L'étanchéité des roches de couverture: l'expérience des pétroliers (M. Lescanne) La caractérisation in situ d'une roche de couverture du point de vue chimique et mécanique: la démarche ANDRA (J. Delay, A. Vinsot) Geochemical and geomechanical reactivity: an experimental approach for caprock integrity (O. Bildstein, M. Jullien) The experimental studies aim at gaining knowledge of the processes and determining the controlling parameters of these processes in order to assess the long term sequestration of CO2. The experiments mainly focus on the confinement properties of the caprock (mostly argillaceous) and on the mechanisms by which CO2 might leak and escape from the depleted gas/oil reservoir or the saline aquifer. To investigate the geochemical reactivity, two main types of experiments were designed to explore the two key scenarios of the performance and safety assessment: batch systems to look at the geochemical reactivity of CO2 (in dissolved and potentially in supercritical form) with the minerals in the caprock, and percolation systems to look at reactive flow of supercritical CO2 (CO2-SC) through chemically or mechanically activated fractures. The first step of this experimental approach is to finely characterise the minerals in the caprock of interest in order to determine the geochemical, geomechanical and petrophysical parameters of the rock and to quantify the confinement capabilities at the initial state, i.e. before alteration. The second step is to perform the same characterisation after alteration to detect any evolution of geochemical, geomechanical, flow and transport parameters in order to assess the impact on the caprock integrity. For the mineralogy, FTIR, X-ray diffraction (XRD) and DTA are the basic techniques used to perform this task. Scanning electron microscopy (SEM) coupled to an energy-dispersion spectrometer (EDS) and TEM are used to look at more precise texture or nature of minerals. In the framework of a French National Research Agency program, the ANR-Géocarbone “caprock integrity” project, a limited zone restricted to the Paris Basin was explored and the callovo-oxfordian geological layers were identified as potential shale and marl caprocks for reservoirs adapted to CO2 sequestration. This choice was also related to the opportunity to study samples coming from an exploited oil reservoir in Saint Martin-deBossenay (SMB, South-East of Paris) and from the underground research laboratory at Bure (URLB, East of Paris). The first site is to become the first French pilot site for the injection of CO2, while the second was set up for the study of deep geological disposal of radioactive wastes (operated by the French waste management agency, Andra). This geological layer shows a remarkable homogeneity over long distances in terms of mineral composition: mainly carbonates (calcite), clays (interstratified illite/smectite and illite), and quartz. The operating mode for the batch experiments is to start reactivity experiments with an initial water composition as close as possible to the formation brine or a composition at equilibrium with the mineral assemblage. This water composition is then modified to match the expected conditions after CO2 injection: equilibration with CO2 gas or CO2-SC. For the batch experiments performed at the CEA in Cadarache with the SMB samples, the rock samples are crushed and reduced to powder (< 500 m), to maximize the reactive specific surface, and then placed into a titanium autoclave where fluids are maintained at constant temperature and pressure. The initial water was synthesised from the composition given by Azaroual et al. (1997). Four sets of experiments were systematically carried out: SMB with brine, SMB with brine acidified with CO2(g), SMB with dry CO2-SC, and SMB with brine and CO2-SC. Two temperatures were chosen for the experiments and maintained at 150 bars during 30/90 days: 80°C which is the temperature at depth of the caprock at SMB, and 150°C which allows for an activation of slow reactions in order to extrapolate results for the long term assessment of the caprock reactivity. For each tests, three replicates of brine were analysed before and after reaction by inducted coupled Plasma – Atomic Emission Spectrometer (ICP-AES). Preliminary results show that the reactivity of minerals with dry CO2-SC is not significant (this result has to be confirmed, see Regnault et al., 2005). At the opposite, significant changes were found when brine was present, resulting in a destabilisation of clay minerals and precipitation of calcium sulfates and carbonates. These results are supported by geochemical modelling which will be further analysed and included in larger scale modelling approaches to assess the impact on confinement properties of the caprock. The same kind of experiments were carried out with pure homoionic Ca-Montmorillonite and with Bure claystone at INPL-Nancy. In this case, the crushed samples are placed into small golden capsule cells along with the brine and dry ice. The conditions of experiment are similar to those described above. Different solutions were used: brine which was previously equilibrated during 1 week with the powder, the same brine acidified with CO2(g), with CO2-SC. A series of experiments are performed at a pressure of 150 bars and a temperature of 80°C and 150°C during 2 and 6 months. For the second type of experiments on original device was designed at the CEA in Cadarache to look at the percolation of CO2-SC through fractured samples (Figure 1). It is composed of a triaxial confinement cell with a series of injection pumps for CO2. The mechanical integrity of the fractured sample is assured by the counter-pressure exerted by a confining fluid on the protective tube containing the sample. At the moment, this device is in a testing phase. In parallel, geomechanical characterisation are also performed in order to determine the deformation properties of the caprock (elastic properties, fracturing strength, …). This type of characterization are planned for pristine caprock plugs and for plugs that underwent degradation due to the interaction with CO2-SC and brine to evaluate the damage caused by geochemical alteration. hc Figure 1. Triaxial percolation cell for reactive CO2 –SC flow-through experiments at the CEA Cadarache. The oven (left pictures) containing the experimental cell (in the middle) allows for thorough temperature control. CO2 gas is injected with a series of pumps (at the left and right of the oven) which maintain the pressure inside the cell. CO2 becomes supercritical due to temperature increase in the heating coil (hc). It then reacts with the fractured sample inside the cell (right picture). Les approches expérimentales au laboratoire intégrité capillaire (D. Broseta, P. Chiquet) La modélisation numérique hydrogéochimique du comportement d'une roche de couverture, extrapolations dans le temps (V. Lagneau) References: Azaroual M, Fouillac C., Matray J.M., 1997. Solubility of silica polymorphs in electrolyte solutions, II. Activity of aqueous silica and solid silica polymorphs in deep solutions from the sedimentary Paris Basin, Chemical Geology, 140, 3-4, pp. 67-179. Regnault O., Lagneau V., Catalette H., Schneider H., 2005. Etude expérimentale de la réactivité du CO2 supercritique vis-à-vis de phases minérales pures. Implications pour la séquestration géologique du CO2. C. R. Geoscience 337, pp. 1331-1339.