View/Open

幾丁聚醣
-
黏土吸附質應用於水中多樣金屬之比較吸附研究
Comparative Adsorption of Cd
2+
, Cu
2+
, Ni
2+
, Pb
2+
and Zn
2+
in Aqueous
Medium onto Chitosan-Montmorillionite Composite Beads
Meng-Wei Wan 1 , Chi-Chua Kan 1 , Sonia Ibarra-Buscano 2,4 , Maria Lourdes P. Dalida 3
1 Department of Environmental Engineering and Science, Chia-Nan University of Pharmacy and
Science, 2 Department of Chemical Engineering, University of the Philippines-Diliman, Quezon City,
PHILIPPINES,
3 Department of Chemical Engineering, University of the Philippines-Diliman, Quezon City,
PHILIPPINES
4 Department of Natural Sciences, Northern Mindanao State Institute of Science and Technology,
Butuan City, Caraga,
ABSTRACT
This study focused on the comparative adsorption of Cd
2+
, Cu
2+
, Ni
2+
, Pb
2+
and
Zn
2+
in aqueous medium using a synthesized novel adsorbent. Composite beads known as chitosan-montmorillionite composite (CMC) and crosslinked chitosan-montmorillionite composite (CCMC) were produced by blending the biopolymer chitosan with natural material montmorillionite in a 1:20 mass ratio. The batchwise adsorption set at 25 ºC, 50 rpm, and pH 4 was investigated based on some experimental parameters including different particle sizes (0.710, 0.500, 0.350 and 0.210 mm), initial metal concentrations ( 5, 10, 50,
100 and 200 mg/l) , adsorbent loading (0.100, 0.200, 500, 2.500 g) and crosslinking with
EGDE in a 1:1 mole ratio. Experimental data showed that adsorption capacity of adsorbent increases while percentage adsorption decreases pertinent to the increase of the initial concentration of the adsorbate. The amount of individual metal ion adsorbed per unit mass of composite beads diminished with high adsorbent amount as manifested by the disproportionate extent of more free binding sites to the inferior presence of diffused divalent ions. Crosslinking enhanced the adsorption efficacy indicating that EGDE preferably linked via the hydroxyl group of pristine chitosan with no change in the amino group. It also decreased the BET surface area of the beads from 74.1171 m
2
/g to 20.0677 m
2
/g. The Zeta potential values of the adsorbent showed a negatively charged surface under the solution pH studied. It displayed a favorable interaction of aqueous heavy metal cation species and indicative as beneficial for adsorption. Evaluation of isotherms using Langmuir and Freundlich yielded good fit with both models signifying that the adsorption sites are non-specific and non-uniform. Data revealed a strong adsorbent selectivity towards Pb 2+ against other divalent metal ions like Cu
2+
, Cd
2+
, Ni
2+
and Zn
2+
chronologically arranged as
: Pb
2+
>> Cd
2+
> Zn
2+
, Cu
2+
>Ni
2+
. Selectivity of heavy metals was due to its different physico-chemical properties like ionic radius, ionic potential, hardness-softness behavior, and electronegativity.
Keywords: Chitosan, Montmorillionite, Adsorbent, Adsorption, Adsorption capacity
第一作者簡介:
萬孟瑋,嘉南藥理科技大學環工系助理教授,專長為廢水處理技術、水質分析、超音波化學、溫泉資
源利用技術與水資。
通訊地址:台南縣仁德鄉二仁路一段
60
號。聯絡電話:
06-2660615
。
1. INTRODUCTION
Over the years, a number of technologies are studied to adeptly remove toxic metals both from water and soils. But common conventional remediation schemes are hindered by its distinct limitations for more effective clean-up operation applications. Prevalent reason is the infeasibility to exterminate trace amount of impurities or even decimate metal concentration to acceptable regulatory standards due to excessive costs linked with their practical relevance. The adsorption technique is one of the preferred methods for removal of toxic contaminants from water. It is noted to be very effective and economical for removal, recovery and recycling of metals from wastewater (Gupta & Bhattachartta 2006).
In this present work, the novel composite adsorbent was synthesized as beads by blending chitosan and montmorillionite. Crosslinking was done to optimize the adsorption efficiency using EGDE as crosslinker. Adsorption mechanism was explored to substantiate its potential for commercialization employing different isotherms and to explicitly interpret the isothermal data. Batch studies were conducted to trap heavy metals from synthetic solutions and establish the selectivity of the material from several ions considered. Use of ICP and discreet chemical analysis are essential for the determination of concentration disparities and metallic solution properties.
Comparative adsorption was investigated using the synthesized adsorbent in a single-ion system with Cd
2+
, Cu
2+
, Ni
2+
, Pb
2+
and Zn
2+
as the targets. The experimental methodology was purposely designed in shaping whether the divalent metal cations are effectively adsorbed onto the composite beads. The working hypothesis is: blending of chitosan and montmorillionite, and chemical modification of the “natbiosorbent” composite will enhance adsorption efficacy resulting to better remediation technology.
Understanding of metal removal is empirical because of the complex structure and composition of beads. In summary, a number of factors influencing the extent of heavy metal adsorption included the bead size, adsorbent loading, solution pH, initial adsorbate concentration, contact time, agitation rate and temperature. In this study, adsorption experiments were carried out at room temperature while adsorbent dosage and agitation rate were fixed at 0.100g and 50 rpm, respectively. Nitrate-based synthetic effluents were used in all experiments controlled at pH 4 and in dilute quantities representing the actual industrial set-up. Low metal concentrations (5-200 mg/l) were used since related study about Cu
2+
adsorption in the same project used very high concentrations (200-2000 mg/l).
2. MATERIALS AND METHODS
2.1 Chemicals and Reagents
Low-molecular weight powdered Chitosan (MW 340.3322 g/mole extracted from crab shells) with 75-85% degree of deactelytion was procured from Sigma-Aldreich.
Commercial Riedel-de Haen Bentonite (as Montmorillionite), NaOH (99%), HCl (fuming
37%), and ICP multi-element standard solution IV from Merck (Germany) were used in the experiments. Cu (NO
3
)
2
.2.5H
2
O, Pb(NO
3
)
2
, Cd(NO
3
)
2
.4H
2
O, Ni(NO
3
)
2
.6H
2
O and
Zn(NO
3
)
2
.6H
2
O were used as source of synthetic effluents. Deionized water (resistance of
18.0 M/cm) was used in all solution preparations and analysis. Weighing was done using the
Ohaus Adventurer
TM analytical balance with readability of 0.0001 g.
2.2 Synthesis of composite beads
Coined as “natbiosorbent” composite, non-crosslinked chitosan-montmorillionite composite (CMC) beads were synthesized based on the previous study conducted in the same lab with slight modification. A single batch of pristine chitosan, used as received without further treatment, was blended with clay particles in a 1:20 ratio (chitosan: clay) forming into beads. Four aspects were vital in the adsorbent preparation classified into chitosan dissolution with HCl; blending of dissolved chitosan with commercial bentonite as montmorillionite; simultaneous neutralization and precipitation with NaOH; and conglomerated physical preparation of beads including drying, grinding, sieving and storage. Crosslinking of chitosan was done to avoid solubility of the adsorbent while preserving surface area to the maximum. In this study, EGDE was used as crosslinker to modify chitosan at a molar ratio of 1:1. The option was sustained by the classification of
EGDE as irritant and less toxic compared to other crosslinking agents. The novel natbiosorbent was called as crosslinked chitosan-montmorillionite composite (CCMC) beads.
2.3 Optimum bead size determination and Adsorption studies
In determining the optimum bead size, a fixed amount of dry beads (3.000 g) of different particle sizes (0.710, 0.500, 0.350 and 0.210 mm) and 50 ml of high concentration synthetic effluents (2000 mg/l) were placed in 50-ml Erlenmeyer flasks. Each flask was sealed with Parafilm to curtail evaporation, agitated in a water bath at 50 rpm for 5 hrs, and sustained at 25
0
C. Subsequent adsorption experiments used 0.100 g of 0.210 mm CMC and
CCMC beads and 30 ml of low concentration adsorbates with exactly the same working conditions. Solution pH was controlled at 4 using 0.1N HNO
3 during batch test for single ion system. After equilibration, the solutions were filtered using Whatman # 40 filter paper complemented by dilution of filtrates into 10 mg/L to match with the ICP matrix.
Quantitative analysis was done using ICP-OES and multi-element standard.
The amount of metal ion adsorbed per unit mass of adsorbent was calculated using the equation:
Q
0
( C
0
C e
) v m
(1)
The percentage adsorption was calculated using the equation:
% Adsorption
( C
0
C e
)
100
C
0 (2)
2.4 Adsorption Isotherms
Basically, the adsorption equilibrium is usually described by an isotherm equation whose parameters express the surface properties and affinity of the adsorbent, at a pre-set temperature and pH (Gupta & Bhattacharyya 2008). The adsorption isotherm therefore is important to describe how adsorbates interact with adsorbents and so is critical in optimizing the use of adsorbents (Juang et al 1997). The two widely used isotherms
Freundlich and Langmuir are usually applied for adsorption studies.
The linearized form of Langmuir isotherm is:
C e q e
1
( bq m
)
(
Freundlich isotherm (Minceva et al 2007): q e
K f
C e n
(4)
1 q m
) C e
(3)
3. RESULTS AND DISCUSSIONS
3.1 Zeta potential and surface area of composite beads
The variation of the net surface charges of chitosan-montmorillionite composite
(CMC) and crosslinked chitosan-montmorillionite composite (CCMC) beads was studied in turn to determine the effect of blending chitosan with montmorillionite. The results of the charge disparity and surface area of adsorbents are shown in Table 1.
Table 1. Zeta potential and surface area of different adsorbents
Montmoril- lionite
Chitosan CMC CCMC
Zeta potential
(pH 4)
BET surface area, (m
2
/g)
-15.9
94.28
-0.228
3.40
-0.390
74.1171
-10.14
20.0677
The net charge of montmorillionite is notably altered upon blending with chitosan which increased from -15.9 to -0.390. The drastic change in surface charge of CMC, which is obviously near that of chitosan, is an indication of a successful coating. Negative sites of montmorillionite are being utilized in binding with chitosan. Conversely, the surprising decrease of structural charge of CCMC denotes that more negative sites are exposed upon crosslinking with EGDE. With this result, it is expected that cationic heavy metals will be favorably attracted to bind with CCMC compared to CMC beads.
With regards to the surface area, it is considered as one of the most helpful microstructural parameters for defining the properties of a porous material like montmorillionite (Bhattacharyya and Gupta 2006). In this study, the measured surface area of the adsorbents is given in Table 1.The surface area of montmorillionite and chitosan shows to be 94.28 and 3.40 m
2
/g, respectively. On blending the two materials followed by chemical crosslinking, the surface area decreased to 74.1171 and 20.0677 m
2
/g, respectively. The low surface area of CCMC beads (20.0677 m
2
/g) compared to CMC beads
(74.1171 m 2 /g) indicates that purely physical adsorption onto the surface is insignificant.
3.2 Optimum bead size
A preliminary experiment using different bead particles was executed to determine the optimum size to be used for subsequent adsorption studies. It was performed in a batch test using a single-ion system. In this study, the uptake of the adsorbate in higher concentration
(2000 mg/l) is decisively influenced by the size distribution of the composite beads.
Reduced particle size (0.210 mm) of both the CMC and CCMC beads exhibited higher adsorption efficiency for all metal ions ranging from 12-32 mg/g except for Zn
2+
which displayed optimum adsorption at 0.350 mm. Generally, it flaunted a remarkable percentage adsorption as depicted in Table 1. This would indicate that there is increased surface occupancy since surface area of the finer adsorbent is obviously higher for the same mass of the larger particles (0.500 and 0.710 mm). For a larger particle array, adsorption capacity decreased faintly due to its limited surface area. The result is noteworthy as preliminary step of evaluating the lofty potential of the synthesized natbiosorbents for industrial applications involving several heavy metals. The order in terms of adsorption uptake can be arranged as
Pb 2+ >Cd 2+ >Cu 2+ >Zn 2+ >Ni 2+ . The ionic size and softness behavior of the heavy-metal cations may have influenced the sequence. The greatest softness value and smallest ionic radius resulted to superior affinity of Pb
2+ ions to the adsorbent. In the case of copper and zinc, lesser hydration of the former may have influenced to its ionic size and greater metal affinity than the latter at higher concentration (2000 mg/l). In acidic condition using CMC beads, the increase of pH levels during the adsorption process of Pb
2+
(pHo=3.75) and Cu
2+
(pHo=4.44) suggested that uptake of heavy metals was subjected to an ion exchange mechanism.
Table 2. Comparative adsorption studies with different bead size (mass=3.00g, adsorbate volume =50 ml, adsorbate conc. =2000 mg/l, t=5 hrs, speed=50 rpm, T=25
0
C, pHo= uncontrolled)
Size
(mm)
Co =2000 mg/l
Chitosan-Montmorillionite Composite (Cmc) Beads
Pb 2+ Cd 2+ Cu 2+ Zn 2+ Ni 2+ pHo 3.75 6.36 4.44 5.64 5.89
0.710
0.500
0.350
Qe, mg/g
% ads pHe
Qe, mg/g
% ads pHe
Qe, mg/g
% ads pHe
29.550
88.87
4.80
30.186
90.81
4.97
30.370
91.38
5.00
21.249
63.86
5.09
21.843
65.58
5.17
21.642
64.99
5.20
14.256
42.86
4.45
15.856
47.69
4.46
16.699
50.25
4.48
14.680
44.12
5.09
15.592
46.90
4.96
16.083
48.31
4.86
12.714
38.16
5.21
15.335
46.03
5.33
14.328
43.05
5.30
0.210
Qe, mg/g
% ads pHe
31.154
93.70
5.01
22.052
66.20
5.00
17.455
52.52
4.53
15.756
47.37
4.88
13.895
41.71
5.36
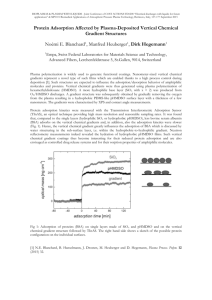
Effects of initial adsorbate concentration and adsorbent loading
An evident change in the extent of heavy metal adsorption was noted by using CMC beads in the comparative adsorption studies at pH 4. At lower concentration, lesser adsorption occurred due to smaller number of metal ions compared to the available adsorption sites. Increasing the metal concentration resulted to more ions in the solution that led to the increase in adsorption capacity as shown in Figure 1. However, percentage adsorption decreased because there is fierce competition of accessible binding sites of the beads at higher concentration. Active sites were already saturated and more ions were left unadsorbed in solution. At lower concentrations (5-200 mg/l) controlled of pH 4, uptake of
Pb
2+
is remarkably higher than the other ions while Zn
2+
and Cu
2+
exhibited similar
behavior. Adsorption trend can be chronologically arranged to: Pb 2+ >>Cd 2+ > Zn 2+ , Cu 2+
>Ni
2+
. This is attributed to the smaller size of Pb
2+ that aids easy access to the binding sites while others are blocked due to larger hydrated radius.
Comparative Adsorption Comparative Adsorption
50.0000
40.0000
30.0000
20.0000
10.0000
0.0000
Ni
Pb
Zn
Cd
Cu
120
100
80
60
40
20
0
Cd
Cu
Ni
Pb
Zn
0 50 100 150 200 250 0 50 100 150 200 250
Initial Concentration (Co), mg/l
Initial Concentration (Co), mg/l
(a) (b)
Figure 1. Comparative adsorption of Pb
2+
, Cd
2+
, Cu
2+
, Zn
2+
and Ni
2+
(a) Adsorption capacity
(Qe) VS Initial conc. (Co), mg/l (b) Percent adsorption VS Initial conc. (Co), mg/l
Apart from complexation, ion-exchange mechanism appreciably influenced the adsorption behavior of all the divalent metal ions due to increase of respective pH levels after equilibration. Pearson’s HSAB theory or softness behavior also holds true in the observed sequence. For individual elements, the softness parameters are Pb=3.58, Cd=3.04,
Zn=2.89, Cu=2.82, and Ni=2.34 (Usman 2008).In terms of anhydrous ionic radius: Pb
2+
=
1.21(1.20), Cd
2+
=0.97, Zn
2+
=0.74, Cu
2+
=0.70(0.72), and Ni
2+
=0.69 (Usman 2008 and
Gomez 2001). The value is inversely related once it is hydrated making Pb
2+
the smallest ion and Ni
2+
the largest.
(a) (b)
Figure 2. Effect of adsorbent loading using CMC beads (a) Adsorption capacity (Qe) VS
Initial conc. (Co), mg/l (b) Percent adsorption VS Initial conc. (Co), mg/l
As depicted in Figure 2, it was noticed that there is similar effect of the adsorbent loading on Pb
2+
, Cd
2+
, Cu
2+
, Zn
2+
and Ni
2+
uptake using the non-crosslinked beads. For
different adsorbent dose (0.100, 0.200, 0.500 and 2.500 g), amount of metal ion adsorbed per unit mass of the beads decreased simultaneously with high adsorbent loading which is contrary to the effect of initial metal concentration. In this case, free adsorption sites outnumbered the quantity of diffused ions and the huge discrepancy resulted to a minimal adsorption capacity. There are more bound ions to the adsorption spots but still leaving a considerable number of these active sites unoccupied. As the quantity of adsorbent was reduced, there was also simultaneous reduction of surface area proportionate to available binding sites hence increased the amount of metal ions attached to it. Analogous observations on this have been reported by other authors. These findings served as edge of using lesser amount of the synthesized adsorbent in actual application thus cost-effective and economical.
3.3 Effect of crosslinking with EGDE
In chemically modifying chitosan with EGDE, equilibrium studies gave different results as reported by many authors. Generally, crosslinking decreased the adsorption capacity of the adsorbent though it depends on the extent of crosslinking. Surprisingly,
CCMC beads enhanced the adsorption capacity of all the heavy metal ions studied particularly in high adsorbate concentration as depicted in tables 3 and Table 4. The result ignified that EGDE preferably linked via the hydroxyl group of pristine chitosan with no change in the amino group as reported by Kurmaev 2002. With this coordination, more binding sites are available for the heavy metals to adsorb. The result also concurred with the measured Zeta potential of the composite beads. These findings served as edge of practically using the synthesized adsorbent in actual application attributed to its enhanced chemical-mechanical strength.
Table 3. Comparative adsorption studies on Chitosan-Montmorillionite Composite Beads
(
C
0 mg/l )
Beads
Data Pb
2+
Chitosan-Montmorillionite Composite
Cd
2+
Cu
2+
Zn
2+
Beads
Ni
2+
5
10
50
100
200
Qe, mg/g
% ads pHe
Qe, mg/g
% ads pHe
Qe, mg/g
% ads pHe
Qe, mg/g
% ads pHe
Qe, mg/g
% ads pHe
1.488
99.30
6.93
2.984
99.66
6.91
14.795
98.93
6.33
28.546
95.25
5.20
47.287
78.97
4.86
1.497
100
7.08
2.981
99.65
6.86
12.090
80.68
6.31
18.073
60.37
5.91
23.844
39.86
5.13
1.497
100
6.32
2.851
95.32
5.30
9.574
64.02
4.84
13.176
44.21
4.81
16.513
27.66
4.71
1.474
98.58
6.36
2.646
93.31
5.96
9.500
63.46
5.38
13.210
40.64
4.92
17.467
29.17
5.02
Note: bead size=0.210 mm, mass=0.100g, adsorbate vol. =30 ml, pHo=4, speed=50 rpm,
T=25
0
C, t=5 hours
1.497
100
6.28
2.646
88.38
5.71
8.267
55.11
5.16
12.143
40.64
4.98
14.823
24.73
4.93
(
Table 4. Comparative adsorption studies on Crosslinked Chtosan-Montmorillionite
Composite Beads
C
0 mg/l )
Beads
Data
Crosslinked Chtosan-Montmorillionite Composite Beads
Pb
2+
Cd
2+
Cu
2+
Zn
2+
Ni
2+
5
Qe, mg/g
% ads pHe
1.476
98.70
-
1.496
100
-
1.496
100
-
1.402
93.66
-
1.493
100
-
10
50
100
200
Qe, mg/g
% ads pHe
Qe, mg/g
% ads pHe
Qe, mg/g
% ads pHe
Qe, mg/g
% ads pHe
2.950
98.73
-
14.837
99.21
-
29.419
98.26
-
54.808
91.62
-
2.997
100
-
13.023
87.08
-
19.696
65.85
-
26.835
44.77
-
2.757
92.08
-
8.943
59.74
-
12.895
43.07
-
17.766
29.67
-
2.631
87.79
-
9.099
60.90
-
13.979
46.69
-
18.491
30.91
-
2.702
90.34
-
10.077
67.45
-
14.096
47.08
-
16.903
28.20
-
Note: Experimental conditions are the same with Table 4.
3.4 Equilibrium isotherms
Experimental data using CMC and CCMC beads revealed that both the Langmuir and
Freundlich models illustrated the adsorption processes fairly well, with regression coefficient (R
2
) >0.900 as shown in Table 5. Freundlich model gives better representation of the isotherm and with the adsorption intensity (1/n) smaller than 1.This an indication of a favorable adsorption which is valid to non-specific adsorption on heterogeneous solid surface (Eren & Afsin 2008). Mostly of the ions behaved this way except for Pb
2+
which demonstrates favorability to Langmuir. The uptake of the metal ions decreased in the order
Pb
2+
> Cd
2+
> Zn
2+ ∼ Cu
2+ ∼ Ni
2+
. These differences in affinity can be due to the existence of dissimilar adsorption mechanisms involving physisorption, chemisorption and complexation as well as ion exchange. Interestingly, the sequence consistently followed the trend in terms of the softness parameter, charge and ionic radius, and ionic potential.
Table 5. Summary of Adsorption Isotherms for comparative adsorption using CMC and
CCMC beads
Metal
Ions
Cd
Cu
Ni
Pb
Zn
2+
2+
2+
2+
2+ beads
CMC
CCMC
CMC
CCMC
CMC
CCMC
CMC
CCMC
CMC
CCMC
K
L
Langmuir Isotherm b
103.0927
4.03226
7.78210
4.40529
6.13402
0.15323
0.59689
0.33480
C max
16.80672
26.31579
13.03781
13.15789
2.89939
3.42466
98.03922
12.34568
5.24109
2.61780
0.23804
0.22945
3.2843
0.40741
0.38836
0.17801
12.18027
14.92537
29.85075
30.30303
13.49528
14.70588
R
2
0.9857
0.9540
0.9870
0.9820
0.9863
0.9950
0.9945
0.9830
0.9872
0.9880
Freundlich isotherm n
3.9170
3.9170
3.2144
3.2144
2.7465
2.7465
2.6015
1.7065
2.8935
2.8935
K
F
6.9678
6.9679
3.6931
3.6932
2.5715
2.5716
13.8643
6.0814
3.2794
3.2794
R
2
0.9996
0.9990
0.9966
0.9990
0.9934
0.9720
0.9503
0.9110
0.9971
0.9950
4. CONCLUSIONS AND PERSPECTIVES
The effectiveness in removing cationic heavy metals from aqueous medium by CMC and CCMC beads depends on many process variables and specific combinations. The results show that the synthesized composite beads have a good potential for use as scavenger of several heavy metals from wastewater effluents. Understanding of metal removal is empirical because of the complex structure and composition of beads thus physical characterization deemed necessary.
Adsorption of five metals in single-ion system of dilute concentration at pH 4 adequately described by the Langmuir and Freundlich isotherms. Binding metal ions largely depends on physico-chemical properties of the metallic specie. It has been observed that metal uptake boosts with the increase in the ionic potential, electronic interaction, hardness-softness behavior (HSAB theory), ionic radius and the electronegativity of the atoms. However, the findings in this present work emerged not to be comprehensive due to dearth of experimental results and test conditions were also inadequate.
It is also emphasized that this investigation was an initial attempt in elucidating the mechanisms involved in the individual adsorption of Pb
2+
, Cd
2+
, Cu
2+
, Zn
2+
and Ni
2+ and that synthesis of composite beads is one of the specific tasks. This is a preliminary endeavor that provides information for more comprehensive researches on metal adsorption using the synthesized novel adsorbent. Generated results from this work are basically interpretative and indicative of adsorption trends which may vary between studies. These are solely partial findings and may not be conclusive to ascertain a valid model for the metal ion interaction.
5. REFERENCES
Bhatttacharyya K.G. and Gupta S.S. (2006) Kaolinite, montmorillionite, and their modified derivatives as adsorbents for removal of Cu (II) from aqueous solution, Separation Debbaudt, A. L., Ferreira, M. L.
Gschaider, M.E. (2004) Theoretical and experimental study of M 2+ adsorption on biopolymers.III
Comparative kinetic pattern of Pb, Hg and Cd, Carbohydrate Polymers 56, 321-332
Eren E. and Afsin B. An investigation of Cu (II) adsorption by raw and acid-activated bentonite: A combined potentiometric, thermodynamic, XRD, IR, DTA study, Journal of Hazardous Materials 151, 682-691.
Gomes P. C. et al (2001) Selectivity sequence and Competitive Adsorption of Heavy Metals by Brazilian
Soils, Soil Sci. Soc. Am. J. 65, 1115-1121.
Kurmaev, E.Z et. al.(2002) Probing oxygen and nitrogen bonding sites in chitosan by X-ray emission,
Journal of Electron Spectroscopy and Related Phenomena 125, 133–138.
Usman, A.R.A. (2008) The relative adsorption selectivities of Pb. Cu. Zn, Cd and Ni by soils developed on shale in New Valley, Egypt, Geoderma 144, 334-343