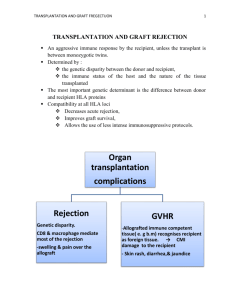
Transplantation Immunology pg. 1 Laura Rayne Slide 1: Title Slide I
advertisement

Microbiology 09/05/2008 Transplantation Immunology Transcriber: Laura Rayne 52:07 (Length of lecture) Slide 1: Title Slide I’m going to talk today about something that’s a little bit different than what you’ve heard up until now. What I am more concerned with is really something a bit more practical in a sense of trying to use the knowledge that we have about immunology to make things work. So this obviously is the subject of transplantation. In a way it is sort of the biological version of engineering. It is like trying to fix a broken car when you have a broken part. So the obvious solution to primates like us, who are tool users, is to replace the broken part. However, like many engineering problems the laws of physics get in your way. Here you shall see how the laws of transplantation, the rules of biology and immunology, can get in your way. So we have to understand how these things work. So when we think about transplantation in general: let’s take it from the perspective of the ancients. Let’s go back to 1500 BC. You’re a slave working for some guy and you’ve done something that has really irritated them so they’ve whacked your nose of. They actually used to do this. They would try things like for example taking a nose from a slave or from another individual and replace it. It never worked. There were other extremely unusual ideas that were tried later, such as in the Middle Ages. For example, it was thought that having bull testicles grafted into you would increase your virility. There were people that actually made money doing this kind of thing. The bottom line is none of this worked. The thing that people found all the way through up until about the 1950s to the 1960s was that if you tried to swap body parts between different individuals it never ever (almost) worked. So why is that? The answer to that comes from our knowledge of immunology and of how immunology works. I have a picture here of Edward Jenner. He came up with an excellent observation and that is that the immune system can be manipulated. He made an observation that one can immunize an individual, although he didn’t know that’s what it was. If you take small amounts of material from Cow pox pustules from people and rubbed it into somebody else through a wound or whatever they became immune to smallpox. So this is one of the first observations that immunity could be manipulated. Slide 2 The story remained relatively unchanged or the next 100-150 years until this gentleman here Sir Peter Medawar, who was a zoologist in 1945 when the Nazis were bombing London they were getting a lot of injuries. So the British medical community became very interested in how to deal with burns and other types of large injuries. Peter Medawar was assisting another surgeon by the name of Gibson. What Medawar observed was one particular patient – a woman who was minding her own business and a V2 rocket comes by and drops an incendiary on her head. She ended up with very extensive burns down her torso to the point where they could not do all of the grafting that they wanted to do. The way they treated burns like this was they would take parts of your skin that were still good and just pinch it between the forefinger and just whack the top off, and take that piece and put it in the wound, and an island of skin would be there and it would slowly grow out and slowly cover the wound. She didn’t have enough skin for that. She had to get some skin from her brother and they transplanted that skin on her and of course that skin was eventually rejected. So they did it again from the brother and Medawar Transplantation Immunology Laura Rayne pg. 2 made this seemingly trivial observation. He saw that the skin that was transplanted on her a second time from her brother was rejected more quickly. Slide 3 Now he noticed that the ability to reject skin more quickly the second time you see the skin (or an antigen) is a characteristic of immunity. So he theorized at that point that maybe the rejection of tissues was an immunologically-mediated response because it happened more quickly. And he later began to do these experiments in rabbits. He published this humongous paper in 1945 called “The Behavior and Fate of Skin Autografts and Skin Homografts in Rabbits”, where he worked out the phenomenology of this entire thing and noticed that there were three characteristics with graft rejection. These three characteristics were inducibility, meaning the response didn’t appear to be there until you put the antigen or the skin there. That it exhibited memory and specificity. These are the cardinal hallmarks of an immune response, so from this observation he concluded that graft rejection was an immunologically-mediated response. Now to us that’s trivial, but in the mid-twentieth century that was huge because it opened up an entire new field. Hence we call him the father of transplantation. Slide 4 So here are the types of experiments he did. Let’s say you have a couple of rabbits. You have two rabbits that are genetically dissimilar. So I colored one red and one blue – I’ll call it strain blue and strain red. So you take a piece of skin from the blue rabbit and put it on the red rabbit, and what you’ll see is that rejection will occur in 7-8 days, roughly. In a normal graft, like say if you were to take a piece of skin from your leg and graft it onto your arm, or like that poor woman who was bombed in 1945 a piece from your leg onto a burn or whatever, what you’ll see is that as you put the graft in place it will sit there. Then the vessels in the graft will begin to reanastamose themselves so you get revascularization. You get healing in 7-10 days. You get a few neutrophils infiltrating the graft and then you have complete resolution. So the graft looks fine. Slide 5 That’s not the case when you actually place a graft from a different individual. What you see is histologically in the image I just showed you of rabbit red being transplanted with a piece of skin from rabbit blue is that you see some revascularization at 3 days or so and there is some variability of when this occurs. But instead of resolution or healing in you see this massive cellular infiltrate come into the graft , and then the graft will actually become necrotic and will eventually slough off and I’ll show you what this actually looks like. This is called First Set Rejection, where you put the graft on for the first time and it is rejected in 7-8 days. This is the observation that Medawar made. Slide 6 Now what happens if you take another piece of graft from the same rabbit that you’ve been using before and you put it on rabbit red again? This was to replicate the observation he made in the Transplantation Immunology Laura Rayne pg. 3 clinic, which was that when the graft was placed on the woman a second time from her brother it was rejected more quickly. Low and behold if you do it in rabbits you see the same thing. The first graft is there. You put the second graft on and it is rejected in about half the time, in 3-4 days. This is a phenomenon he called Second Set Rejection. This is evidence of immunologic memory, meaning that it remembers its prior exposure to the antigen and in doing so reacts more quickly to that antigen or skin. Slide 7 So just like I showed you a moment ago you see that the graft is rejected and you see a mass of cellular infiltrate, and he described this histologically as well in the original article. Slide 8 Now evidence of specificity comes from the fact that if you take a piece of skin from a third rabbit known as a third party - so you have this same rabbit here and you’ve done teo grafts on him and you see that the first graft is rejected in 7-8 days. You put a second graft on and it is rejected in 3-4 days. At the same as this one you put another graft on from a different genetically unrelated rabbit who has no relationship to either one of these rabbits. This shows evidence of First Set Rejection. So it is rejected in 7-8 days. So what this is telling you is that this rabbit here is able to distinguish between a graft from this rabbit and a graft from this rabbit. So it’s exhibiting a response that we call specific. This was the basic observation that Medawar made and that is that graft recognition is a) an immunologically-mediated response and b) is related to the genetic constitution of the individual. That is, it has to do with the genetic relatedness of individuals. Slide 9 When you look at a Second Set Rejection here what you see is that instead of seeing a healing in phase like you see with first set rejection what you see is this neutrophil and lymphocyte riot that occurs almost immediately within 3-4 days. You don’t even get healing in and the graft is rejected and you get thrombosis and necrosis very quickly. So it’s a fairly violent response. Slide 10 We’ve developed a terminology for this that is essentially built around the genetic relatedness between individuals. The first item on the list that you see up there are called autografts meaning self. These grafts here are from one site to another on the same individual. An isograft is between two genetically identical individuals so this would be between two identical twins or in my case I do grafts between genetically identical mice of the same strain. Slide 11 An allograft is between two genetically distinct individuals of the same species, so this is the grafts between the rabbits. What Medawar did was allografts. What we do clinically when we swap kidneys and hearts and livers and lungs between people are allografts. The Xenograft is between Transplantation Immunology Laura Rayne pg. 4 different species so if you were to have a duck-billed platypus and you wanted to do a graft from that animal to a rabbit you could that, or from pig to man which people would love to do. Slide 12 If you were to look at a mouse and see what this actually looks like. This is the back of a black mouse. What you see is a skin graft that has been placed on a mouse. This is the very beginning of rejection here. The key feature that you start seeing from a gross morphological point of view is you start seeing these little scabby portions appearing on the graft. Slide 13 Then after a little while longer you see that the graft completely scabs over and then falls off. In this animal here you see a rejected graft. So from a gross morphological point of view that is what it looks like. Slide 14 This is the basic phenomenology of it all. It works this way if you transplant skin, hearts, lungs, liver all those kind of things that are clinically important to us and clinically important to you if you have end-stage organ disease with no other solution available. That same phenomenology applies. The only thing that is really different in an untreated individual is the timing. If you have a skin graft and it gets rejected, the skin falls off. If you have a heart graft and it gets rejected you drop dead, but the end results are that the graft stops working. The question is how does it work? Medawar made this observation that there are all these odd looking cells in the graft called lymphocytes in huge numbers. In the mid-twentieth century they didn’t know what those were for. So you design an experiment that asks the question “Is graft rejection mediated by T cells?” In a lab like this you will go into your mouse room and grab a mouse, we’ll call him strain B, and we’ll do the same type of experiment that Medawar did. We’ll take a graft from another stain, strain A, and put it on there. We’ll look in 14 days and in this case we see First Set Rejection. The graft is rejected and we see necrosis, just like I showed you in the picture there. If you were to take a second graft on the same mouse and do a second skin graft with strain A just like Medawar observed you’d see that it would reject in about 6 days. But we want to know if T cells have anything to do with it. You take this mouse and you take the spleen out, and you purify the T cells, and you inject it into another mouse that has never seen or been transplanted with a piece of skin graft. Then you do a transplant on that animal. What you discover is that the graft is rejected with Second Set kinetics. What that means is that you used the T cells to transfer the memory of the exposure to the graft. Hence, T cells are involved. A very simple yet very nice experiment that shows exactly how this works. Slide 15 Transplantation Immunology Laura Rayne pg. 5 You know that a T cell isn’t a T cell. We can sit there and subdivide cells and decide which T cells are involved in graft rejection. We can do another experiment: what if you take a mouse and wipe out all the CD4+ cells or CD8+ cells and then observe what happens to graft rejection? This is a classic survival plot. What it shows is the % of surviving grafts vs. time. When an animal (or more than one) rejects in your group in a given time the survival goes down in a stair-step like fashion. If you just take some animals shown in black as the control and just do your transplant as you should, you’ll see that they are all gone by 15 days. But this is sort of variable. You don’t do grafts and then on day 10 everybody just rejects. It works where you see a continuum. Now you take some other animals and you do the grafts on them and you inject them with an anti-CD8 monoclonal Ab. What the Ab does is wipe out all the CD8+ cells. Now you have an animal with mostly only CD4+ cells. What you find is these animals reject at the same rate. There is really no difference. That tells you that CD8+ cells are not absolutely required for rejection. Now you do the opposite experiment where you treat them with anti-CD4. So this wipes out all the CD4+ cells leaving only CD8+. In this case what you see is the graft survival is much longer. That means that they will reject if only CD8+ cells are there, but they don’t reject as quickly. It is clear that CD8+ cells aren’t required but they do help in rejection. When you wipe them both out rejection is vastly extended. The fact that it occurs at all is because when you inject a mouse with anti-CD4 and anti-CD8 monoclonal Abs you wipe them out but the animal begins to repopulate after a while. New cells come out of the thymus, they repopulate the external lymphoid organs and they come back. When they do eventually the animal will reject unless you keep treating it continuously. So we can see that CD8+ and CD4+ cells are important in rejection. It is a T cell mediated response. CD4+ cells are absolutely required for this to occur. CD8+ cells are helpful in this response but are not absolutely required. So we get an idea of the mechanisms involved here. We also know that transplant rejection is also related to the genetic relatedness between donor and recipient. Clearly it is a genetically-driven response. Slide 16 There were a number of observations that were made during the 1920s and 1930s using inbred strains of mice. They came up with these laws of transplantation. So if you’re working with inbred strains of mice, which are mice that have been bred over the last hundred years or so and within a given strain they are almost all genetically identical. You can swap transplants between those inbred strains within those strains and those grafts will take. So the law is that transplants between individuals of the same inbred strain will succeed. Transplants between inbred strains are rejected. It is just like people. If you have a transplant between identical twins it will succeed. If you have transplants between two different individuals who are not twins it will be rejected. Here’s something that is less intuitively obvious: if you do a transplant from a Transplantation Immunology Laura Rayne pg. 6 parent to an F1 (F1 being the progeny of the two parents) that transplant will succeed but the reverse will fail. I show you this because it illustrates how genetics drives graft rejection. Slide17 If we look at two strains, how would this work exactly? Remember, MHC gene products are involved in restriction of antigen recognition at the T cell receptor (not sure what he means by this). Strain b mice have MHC complex haplotypes called b, one allele from each parent. The same for this strain called k, again receiving one copy from each parent. Each of these parents has one of each allele. They have F1 that inherits an allele from each parent so this mouse now has a b allele and a k allele. So if we think about how the genetic relatedness influences this, we find that the more similar you are, the less likely you are to reject. Over the course of the years we have discovered that the main driving force behind graft rejection genetically is the MHC complex (repeats this). What we see here is this inheritance pattern of receiving the MHC genes then will influence how this individual behaves to transplants. Let’s go back and look at the rules again (previous slide). Look at the third one. If we look at the way that these genes are inherited we see that if there are transplants from the parent to the F1, the F1 has the same alleles that the parent does. This mouse which has b, sees b as self, so it doesn’t reject the graft. What if we go in the opposite direction? We’d go from b/k to b/b. Well the b mouse has no experience with k and so it sees k and therefore rejects the graft. Transplants from the parent to F1 will succeed, but the reverse will fail. It is simple recognition. Of course the other question that someone will think of eventually is why don’t we do this in people? The reason is that people aren’t homozygous like inbred mice are and the MHC is a multi gene locus. So an individual human will have so many combinatorial possibilities that these rules work at one locus or in homozygous individuals, but if my child needs a kidney from me there is no guarantee I can donate that kidney to them and have it work, although there is matching that can take place. Slide 18 When we look at humans, we see how the locus looks. In humans there are a number of different histocompatibility genes that control graft rejection – MHC Class II and MHC class I are the most important of those. These molecules have their own characteristics and distribution throughout the body. Remember that class II are primarily present on APCs and B cells, and Class I are ubiquitously distributed throughout the body. Slide 19 So if we’re going to do a transplant on somebody in the clinic , it is driven by the degree of genetic relatedness between one individual and another. This is the key. When we do clinical transplants in people we expend a great deal of effort trying to determine the genetic relatedness between a potential donor and a recipient. Of course, you don’t want to transplant an organ that you think will be rejected immediately. You’re just going to kill them. One of the oldest methods we have Transplantation Immunology Laura Rayne pg. 7 used to distinguish between two individuals is to do histocompatibility typing. What one will do is take cells from the donor and the recipient (frequently lymph node cells). You grind them up and separate the cells and seed them into different little wells. You place those cells in the plates here and you use Abs that you have raised against various MHC antigens. You expose the cells to specific Abs and if the cells have those antigens on their surface, the Abs will bind to it and you can determine whether that Ab is there by adding complement. Complement in combination with Ab bound to the surface will begin to blow holes in the cell membrane. You can then treat these cells with a vital dye (for example trypan blue) that goes into the cell if the cell has holes in it. Dead cells become blue, live cells remain white. In the case where the Ab does bind the cells become leaky. Slide 20 What you can do then is run a panel. You’re using Ab to different HLA antigens. Here you have your recipient. You know that this person is say A1 and A7 (2 alleles, one from each parent) and donor 1 has A1 and A7 – a perfect match. Donor 2 has A2 and A3. Which donor would work best? Obviously the first one would be the one that you would choose, all other things being equal. This is how many of the decisions are made, particularly for kidney transplantation. These assays are actually done using beads and other types of reagents now. Slide 21 You can also do another type of assay called a Mixed Leukocyte Reaction (MLR or MLC) where one can take for example, a spleen from two different strains of mice called blue and red, and if you simply mix them together in a culture disk these cells will proliferate wildly in response to each other. You can prove that this response is related to MHC because if you take two spleens from two inbred strains of mice, say red and red or blue and blue, nothing happens. But if you take red and blue and put them together and look for proliferation, a common way to do that is to use a DNA precursor like thymidine which is labeled with tridium. What happens is when cells multiply they make DNA of course and they take up this DNA precursor and become radioactive. So you can tell if the cells are proliferating. You can see that simply mixing leukocytes together can cause them to proliferate rapidly if the two individuals are genetically unrelated. You can tell if one individual or another is proliferating by simply irradiating one of these for example with 2000 RADS. It renders them unable to proliferate but the can stimulate just fine. Slide 22 So now you transplant an organ clinically and what happens? Acute rejection appears anywhere from about 10 days on post-transplantation and can occur anytime after, even 20 years later. It is primarily cellular and you see a massive infiltration of macrophages and lymphocytes and you obviously see decreased graft function. Slide 23 Transplantation Immunology Laura Rayne pg. 8 I work in cardiothoracic surgery so I am a little bit biased toward heart. This is a histological section of heart muscle stained with H&E. You can see the heart muscle is largely a pink material. Nuclei show up as a blue color and what you see is that this graft has some infiltrate that you don’t normally see in healthy heart muscle but there is not much. The cytoarchitecture is largely intact. Slide 24 When we look at a graft that is rejecting however, what we see is a much more serious infiltration of tissue. This is a little bit higher magnification. One even sees eosinophils in this particular sample and you see a large infiltrate of lymphocytes, macrophages, neutrophils and a marked disruption of the cytoarchitecture of the tissue. (As an aside, we diagnose heart transplant rejection in individuals by biopsying the heart. If you wait until they begin to have compromised graft function they have this tendency to drop dead on you before you know anything is happening. So what we do is take a biopsy sample and do histological sectioning. That is where this is from.) The primary indicator is signs of this type of infiltration. It is treatable. We have a number of immunosuppressant drugs to do that, particularly steroids. Slide 25 Another type of rejection that we call chronic rejection is very different. It is characterized by a slow and steady decline in graft functions and what one typically will see is a progressive occlusion of vessels and passageways within the graft. In the heart it would be the coronary arteries. In the lungs you’ll see it along the trachea and the bronchi. In the lungs they call this bronchiolitis obliterans. It seems to be the result of cellular and humoral mechanisms. That really means that we don’t know. We know cellular responses and antibodies are involved but we don’t know exactly how. And in fact we really have no satisfactory treatments for chronic rejection. Very often this will progress over time, usually a period of years, and there isn’t a whole lot we can do about it. Slide 26 So what does it look like exactly? This is in a mouse model of chronic rejection done in the laboratory. We do heart transplants and kidney transplants in mice in an attempt to model what is happening in humans. If we look at a normal coronary vessel, what we see here is the vessel wall is composed of a series of layers consisting of the inner layer, the intima, and layers of elastin and connective tissue. In a heart that is undergoing chronic rejection what you instead see is the intima is right here and you see this hyperplasia that occurs inside the intima such that the vessel diameter is now reduced. This occlusion will progress to the point where this vessel will no longer carry blood. The heart will eventually stop working. Slide 27 The hyperacute rejection is the atomic bomb of transplant rejection. It was first observed clinically between individuals who were blood type mismatched or they had some other reason that resulted in preexisting antibodies occurring in the recipient. Hyperacute rejection is caused by Transplantation Immunology Laura Rayne pg. 9 preexisting antibodies. What you see is when you’re the surgeon and you’ve transplanted this kidney and you pull the cross clamp off to allow the blood flow to go to the kidney, you see the kidney get nice and pink and it begins to function and produce urine after a while. Now it’s different. You pull the cross clamp off and the thing doesn’t pink up. Instead what you see is hemorrhagic loci appearing on the outside of the organ and the thing will actually turn black. You can take a rat heart out, for example, and hang it on a stand and perfuse it with oxygenated buffer and that thing will just sit there and beat merrily away as long as you want. Then you switch it to a blood supply from a different species of animal that has preformed antibodies against that rat heart and within 30 seconds it stops doing any useful work at all and in 20 minutes all you see is this barely quivering hunk of black meat. So you obviously don’t want this to happen clinically. How does it work? Well it is due to preformed Abs existing in the recipient. You release the cross clamp, blood surges into the organ and brings this preformed Ab into the organ. The Ab immediately begins binding to the endothelium on the capillary walls, fixes complement and begins blowing holes in the endothelium. Of course, platelets and neutrophils begin to adhere to the walls of the endothelium and the vessel gets blocked off. This can occur within minutes, resulting in the total destruction of the organ. It is a very, very rare phenomenon. We don’t really see it anymore unless you’ve screwed up in a really gross way. Student question: “Is there a way to tell when that’s going to happen?” Answer: There is. In fact, we expend a fair amount of effort making sure that won’t happen. There are ways that you can test for preformed Abs. One way is called the cross-match. Not tested on. The rest of the material will not be tested on because he did not have time to cover it. Transplantation Immunology Laura Rayne pg. 10