guide for Authors
advertisement

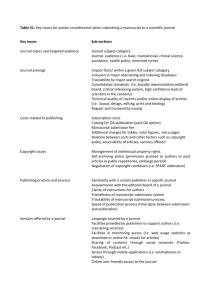
Author Guidelines 1. General Korean Journal of Oriental Physiology & Pathology (ISSN number : 1738-7698) is a bimonthly, international journal for the publication of original research papers, reviews, clinical reports, and short communications on research of Oriental Medicine including Eastern Medicine and Traditional Korean Medicine. Manuscript Submission. Korean Journal of Oriental Physiology & Pathology operates an online submission. All papers must be submitted via the online system. Submission of a manuscript will be held to imply that it contains original unpublished work and is not being submitted for publication elsewhere at the same time. File types. Preferred formats for the text and tables of your manuscript are .doc, .rtf. Figures must be provided in .tiff, jpg, or .eps format. Upon acceptance, author(s) must supply by e- mail as a scan file: a Copyright Transfer Agreement with original signature(s). If the manuscript contains extracts, including illustrations, from other copyright works (including material from on-line or intranet sources) it is the author's responsibility to obtain written permission from the owners of the publishing rights to reproduce such extracts. Manuscript Style. The language of the journal is English or Korean. Please ensure that your manuscript has been checked by an English (or Korean) language expert if there is concern for grammatical or other errors. All submissions must have a title, be double-line spaced with type no smaller than 12 point, and have a margin of 3cm all round. Tables and figures must be on separate pages after the reference list, and not be incorporated into the main text. Figures could be uploaded as separate Image files. Original Papers. These should not exceed six printed pages (where one page comprises 800 words or the equivalent in illustrative and tabular material). Short Communications and Clinical reports. These must be complete, self-contained papers, and not preliminary reports. These should not exceed three printed pages including a maximum of three figures and/or three tables and 20 references. To exceed the limit may delay acceptance or publication of the paper. Reviews. The body of a review article should be a comprehensive, scholarly evidence-based review of the literature, accompanied by critical analysis and leading to reasonable conclusions. Wherever appropriate details of the literature search methodology should be provided, i.e. the databases searched (normally Medline and at least one or two other databases), the search terms and inclusive dates, and any selectivity criteria imposed. Wherever possible, use primary resources, avoiding “Data on File”, “Poster” or other unpublished references. 2. Draft Details Title page. The title page must list the full title, short title of up to 60 characters and names and affiliations of all authors. Give the full address, including email, telephone and fax, of the corresponding author who is to check the proofs. Research Grant. Include the name(s) of any sponsor(s) of the research contained in the paper, along with grant number(s). Abstract. Supply an abstract of up to 250 words for all articles. An abstract is a concise summary of the whole paper, not just the conclusions, and is understandable without reference to the rest of the paper. It should contain no citation to other published work. Keywords. Include up to six keywords that describe your paper for indexing purposes. Introduction. A concise introduction is required of the background to the subject, its significance and its relationship to earlier works, with references. Materials and methods should be presented with clarity and detail. State the original and important findings in the results. Illustrate these with figures or tables where necessary but keep these to a minimum. Results and discussion may be combined as one section. Discuss the principal conclusions drawn from the results and their important implications. Conclusions. This must summarize the main paper. Ensure that extrapolations are reasonable and that conclusions are justified by the data presented, and indicate if the study design can be generalized to a broader study population. Convention on biodiversity. Authors must indicate that they have obtained authority to access plant samples (other than freely available commercial crops or herbal products) used for research and that this has been authorised by the appropriate agent of the government of the source country as required under the framework of the United Nations Convention on Biodiversity. Botanical aspects. For papers relating to crude plant extracts, the method of extraction and the yield of dried extract as a percentage weight of the starting fresh or dried plant material must also be stated. Use Chemical Abstracts nomenclature for chemical names and structures. Use proper or proprietary names with caution. Common acronyms for biomedical names are acceptable but define all others when first mentioned. Define abbreviations when first mentioned and do not use in the title or abstract. Define non-standard units. Acknowledgements. Keep acknowledgements brief and place them at the end of the paper. Reference style. References should be given in the Vancouver style. Citation in the text is indicated in a form of superscript by Arabic numbers in brackets: (1), (2,3) etc. All references must be complete and accurate. Korean Journal of Oriental Physiology & Pathology uses Index Medicus Style abbreviations for journals cited. For correct abbreviations visit http://www.ncbi.nlm.nih.gov/entrez/jrbrowser.cgi. References should be listed in the following style: Journals: Wright CW, Phillipson JD. Natural products and the development of selective antiprotozoal drugs. Phytother Res. 4:127-139, 1990. Books: Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas (2nd edn). Berlin Heidelberg;Springer-Verlag. 30-38, 1996. Chapters in Books: Kips RH. Environmental aspects. In Pesticide Application: Principles and Practice , Haskel PT (ed). Oxford;Oxford University Press. 1-34, 1985. Electronic Resources: Lin A-S, Shibano M, Nakagawa-Goto K, Tokuda H, Itokawa H, Morris-Natschke, SL, Lee K-H. Cancer Preventive Agents. 7. Antitumor-Promoting Effects of Seven Active Flavonolignans from Milk Thistle (Silybum marianum) on Epstein-Barr Virus Activation. Pharm Biol [Online] 2007;45:735-738. Available at: http://www.informapharmascience.com/doi/abs/10.1080/13880200701585592. Accessed on 12 April 2009 Tables. Tables should be used only when they can present information more efficiently than running text. Care should be taken to avoid any arrangement that unduly increases the depth of a table, and the column heads should be made as brief as possible, using abbreviations liberally. Lines of data should not be numbered nor run numbers given unless those numbers are needed for reference in the text. Columns should not contain only one or two entries, nor should the same entry be repeated numerous times consecutively. Tables should be grouped at the end of the manuscript on separate pages. Illustrations. Upload each figure as a separate file in .tiff, .jpg, or .eps format. All illustrations must be supplied at the correct resolution (300 dpi or higher). Legends or captions for figures should be listed on a separate page. Copyright. To enable the publisher to disseminate the author's work to the fullest extent, the corresponding author must sign a Copyright Transfer Agreement, transferring copyright in the article from the author to the publisher, and submit the original signed agreement with the article presented for publication by e-mail to the Content Editor. Conflict of Interest. All authors must declare financial/commercial conflicts of interest. Even if there is none, this should be stated in a separate paragraph following on from the Acknowledgements section. This is a mandatory requirement for all articles. 3. Ethics and Consent Do not use patients' names, initials, or hospital numbers, especially in illustrative material. Identifying information should not be published in written descriptions, photographs, and pedigrees unless the information is essential for scientific purposes and the patient (or parent or guardian) gives written informed consent for publication. Informed consent for this purpose requires that the patient be shown the manuscript to be published. Manuscripts including animal experiments or clinical trials must be conducted with approval by the local animal care or human subject committees, respectively (see below). All manuscripts, except reviews, must include a statement in the Introduction or Methods section that the study was approved by an Investigational Review Board (Human Studies Committee or Ethics Committee or Animal Care and Use Committee), if applicable. Authors who do not have formal ethics review committees should include a statement that their study followed principles in the Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/index.html). When a product has not yet been approved by an appropriate regulatory body for the use described in the manuscript, the author must specify that the product is not approved for the use under discussion or that the product is still under investigation.