preparing your predoctoral fellowship application form
advertisement

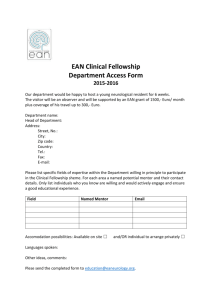
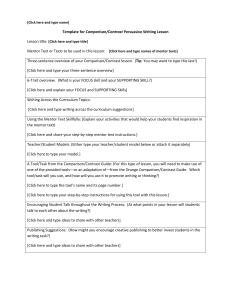
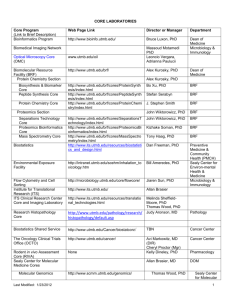
Instructions for preparing your pre-doctoral funding application Page 1 of 2 PREPARING YOUR PREDOCTORAL FELLOWSHIP APPLICATION FORM The same form is used by all three Infection and Immunity graduate training grants to evaluate applications for funding submitted by UTMB graduate students. These three Programs maintain separate review processes, but they do communicate to assure the best use of our limited funds. GENERAL INSTRUCTIONS: Please read and follow the instructions carefully. Failure to follow the application format and length limits or failure to submit all required components of the application (including the Mentor’s letter of support – see Predoctoral Mentor Letter instructions) will result in the application being returned without review. Prepare the application single-spaced using the form and format provided. Form pages have been left "unprotected" to allow you to include your own text, insert graphics, diagrams, tables, etc. You must develop the content of your application yourself — it must not be the work of your mentor. Of course, you may receive input from your mentor, but it must not contain “cut-and-pasted” material or other materials (manuscripts, grant applications, reports) prepared by your mentor and/or his collaborators. Format Specifications: Follow the format instructions on the application form. There is no allowance for appendices, except in the case that http sites are not readily accessible to key references that the applicant, mentor, or key collaborators have produced. MCLAUGHLIN-SPECIFIC REVIEW PROCESS: The T32 training grants have specific review processes that may be obtained through their Program Directors, Dr. Scott Weaver or Dr. Stephen Higgs. For McLaughlin Fellowship applications, review will follow the three specific weighted criteria outlined in the attached “Sample McLaughlin Pre-doc Review.” The review panel will consist of members of the McLaughlin Committee, with additional outside reviewers solicited by the Committee from UTMB faculty. All reviewers review all applications, but individual reviewers serve as primary and secondary reviewers for each application. All applications are discussed in a single review session. Review panels will not include any mentors of applicants under review. To help ensure that McLaughlin Predoctoral Fellowships support the most meritorious candidates from throughout the UTMB infectious diseases and immunology research community, the following policy was established in FY 07: Multiple applications for predoctoral fellowship support may be submitted by applicants from a single UTMB laboratory, but only one fellowship will be awarded to any single laboratory each year. SPECIFIC APPLICATION INSTRUCTIONS: Item 1: Title of Project - Do not exceed 81 characters, including the spaces between words and punctuation. Choose a title that is specifically descriptive, rather than general. Items 2-6: Self explanatory Item 7: Give a description of your research experience, scientific meetings attended, abstracts, publications, etc. and explain their relevance to your application. Item 8: List any awards you have received and their relevance to your application. Also, list any lab rotations, and give the name of your Mentor and duration of time spent with him/her and any achievements received under their direction. Item 9: Please indicate all fellowship sources that have supported you at UTMB. Item 10: Please complete the requested essay. infectious disease research. It will be used to help determine your commitment to Item 11: Please indicate if this application is a resubmission. If it is, please provide the title of your original application and supply the requested “introduction” to your revised application. UTMB Intramural infectious diseases predoctoral training grant application instructions. Last updated 12/07 Instructions for preparing your pre-doctoral funding application Page 2 of 2 Item 12: Research Plan – The Research Plan should include sufficient information needed for evaluation of the project, independent of any other document. Be specific and informative, and avoid redundancies. Organize Items a-d of the Research Plan to answer these questions: 1. What do you intend to do? 2. Why is the work important? 3. What has already been done? 4. How are you going to do the work? DO NOT EXCEED 2 PAGES, SINGLE SPACED, (ARIAL 11-POINT), INCLUDING FIGURES AND TABLES, FOR 12a-d. Item 12a: Specific Aims – State concisely and realistically what the research described in this application is intended to accomplish and/or what hypothesis is to be tested. List the broad, long-term objectives and what the specific research proposed in this application is intended to accomplish. Item 12b: Background and Significance – Briefly sketch the relevant background leading to the present application, critically evaluate existing knowledge, and specifically identify the gaps that the project is intended to fill. State concisely the importance and health relevance of the research described in this application by relating the specific aims to the broad, long-term objectives. Clearly identify the relevance of the research to infection and/or immunity, and if relevant, to emerging and reemerging infectious diseases and biodefense. Item 12c: Preliminary Studies Use this section to provide an account of preliminary studies pertinent to the application information and/or any other information that will help establish the experience and competence of the applicant to pursue the proposed project. The http sites of key complete references should be included in item 13. (If references are not accessible through the UTMB ejournal list, please supply as a separate pdf file). Item 12d: Research Design and Methods – Discuss concisely the experimental design to be used to accomplish the specific aims of the project. Include how the data will be collected, analyzed, and interpreted. Discuss the potential difficulties and limitations of the proposed procedures and alternative approaches to achieve the aims. It is recognized that key preliminary data may not have been produced by the applicant; if this is the case, please so indicate. Item 13: Literature cited – Include critical literature citations referred to in section 12a-d, include http links for up to 3 key citations from applicant or mentor. There is no page limit for this section, but be succinct. Item 14: Applicant’s BioSketch (4-page limit) – Complete an NIH-style BioSketch following the NIH instructions (http://grants2.nih.gov/grants/funding/phs398/phs398.html). Item 15: Mentor’s BioSketch (4-page limit) – Insert a BioSketch from your primary mentor. This BioSketch must include research support information. Item 16: Grade transcripts (undergraduate and graduate): Scan of official documents is sufficient Item 17: GRE scores: Scan of official document is sufficient. SUBMISSION INSTRUCTIONS: Render your entire application into a single pdf file. Name your file: use your last name and “predoc” (e.g., ABSmith-predoc.pdf). Submit to McLaughlin.IHII@UTMB.edu prior to 5PM March 17, 2008. UTMB Intramural infectious diseases predoctoral training grant application instructions. Last updated 12/07