here
advertisement

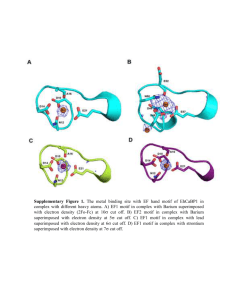
Details of intra-molecular changes during signal peptide and nucleotide binding to SRP54 Signal peptide binding into the hydrophobic groove in SRP54M opens the finger loop and adjusts the entire N-terminal part of the M domain presumably via four conserved hinge motifs (Fig. 1c). This involves a tilt of helix M1 and a movement of helix M1b, presumably via a conserved tandem ‘GP’/‘PG’ motif, which may lead to a structure as observed in the SRP54M of T. aquaticus (Keenan et al., 1998). A hydrophobic contact between the N domain (LN34) and the M domain (M1M1b) would be directly influenced or even lost by this rearrangement. This allows for movements of the M domain relative to the NG domain as well as of the N domain relative to the G domain. The first one involves the conserved ‘LGMGD’ linker motif in the loop connecting the G and the M domain. This rotation would also open the proposed interaction between the G domain and RNA helix 8. The second one is a rotation around helix N4, which is observed in T. aquaticus (about 6°) depending on the nucleotide load (Ramirez et al., 2002) (Fig. S1). This rotation moves the conserved ‘ALLEADVN’ motif in the NG domain interface. The motif is intimately linked to a conserved ‘GQ’ motif located at the N-terminus of helix G3 and to helix G4 including the ‘DARGGA’ motif (Figs. 1c and S1). The concerted movement is enabled by (I) a conserved extended hydrophobic patch in the NG interface (Montoya et al., 2000), (II) a packing defect of helix G4 towards the G domain (Ramirez et al., 2002), (III) a ‘spring-like’ elongation of the N-terminal half of helix G3 (induced by a switch from a to a 310 helix) (Freymann et al., 1999, Nature Struct Biol, 6: 793–801), and (IV) the missing side-chains in the glycine residues in the ‘DARGGA’ motif (only last item shown in Fig. S1). The observed N versus G rotation preserves this inter-domain contact, but has important consequences on the conserved G-4 motif (‘TxxD’), which communicates this conformational change to the GTP binding site. The important consequence is the repositioning of the strictly conserved aspartate in the G-4 motif, which binds to the Watson-Crick face of the guanine base and thereby provides nucleotide specificity. Only in the nucleotide bound forms (with the N domain rotated), the aspartate is close enough to complete the GTP binding pocket and to form two hydrogen bonds with the nucleotide. At the same time, the GTPase G-5 motif (‘closing loop’) folds over the nucleotide base (not shown) and the high affinity GTP binding state of SRP54 is established. The changes in the NG domain interface are as well communicated to the G-2 and G3 consensus motifs located on the other side of the nucleotide binding pocket (Fig. S1). Most importantly, the G-2 (‘DxxR’) motif is responding to nucleotide binding in the presence of Mg2+ and gets significantly destabilized by the interruption of several side chain interactions (Freymann et al., 1999). A salt bridge between the aspartate of the G-2 motif and the arginine in the G-3 motif (‘DT(A/S)GR’) is disrupted. At the same time, the invariant glutamine in the G-1 motif (‘GLQGxGKT’) looses its connection to another invariant arginine in the G-2 consensus motif. Thus, the whole region is reorganized and SRP54 is now in a favourable conformation to interact with its receptor (see (Egea et al., 2004; Focia et al., 2004)). Differences for Crenarchaea The molecular model presented for how signal peptide binding influences the nucleotide load during the first step of the SRP cycle applies to the eukaryotic, eubacterial and the euryarchaeal SRP. The second subclass of Archaea, the Crenarchaea, however might be different. The comparison of the known NG domain apo structures of SRP54 from Crenarchaea (Montoya et al., 2000; Rosendal et al., 2003) with the one from the Eubacterium T. aquaticus (Freymann et al., 1997) shows significant differences. In Crenarchaea, a unique dipeptide insertion (‘GY’ or ‘GH’) in the so-called GTPase switch II region seems to be responsible for a large overall re-organisation of the whole NG domain (Fig. S1). The apo conformation of the crenarchaeal NG domain already resembles a nucleotide bound state with the N domain rotated as described above. The rotation is even five times bigger, which seems to be enabled by an extended ‘glycine slide’ in the ‘DARGGA’ motif (‘DARGGG’). This implies that in Crenarchaea GTP binding to SRP54 may obey different rules than in the eubacterial homologs.